Physiology Advances and Reflections on the Integrative Physiology of Exercise and Cardiac Protection
-
摘要:
从心脏自身、远隔器官和中枢调控等方面系统梳理心脏从运动中获益的相关文献,探讨其机制,对促进大众和心脏病患者认识、理解和信任运动对心脏的保护作用,推动基础研究与临床应用的融合,具有重要的理论和现实意义。梳理文献发现,运动可直接降低罹患心脏病的风险,改善心脏结构和损伤,并优化心脏代谢效率。同时,运动通过促进骨骼肌、肾、肝和脂肪等远隔器官或组织分泌“运动因子”,调节肠道菌群及其代谢产物,保护心脏。基于中枢多器官调控网络,运动可通过中枢调节心脏自主神经活动,优化心血管反射敏感性,并通过“脑-心”轴、“肠-脑”轴和“肝-脑”轴等多轴途径改善心功能。提出心脏运动康复从基础研究转化至临床应用过程中的机遇与挑战,“运动模拟物”开发、实验动物模型、远程康复的安全性和高效精准运动指南等方面均值得高度关注。同时,关注神经心脏病学发展和“脑心同治”“脑心共研”“脑心同康”对揭示运动的“脑心同康”机制具有重要意义。
Abstract:This study reviewed the literature on cardiac protection from exercises in terms of the cardiac, remote organs and central nervous system. The mechanism is explored, which has significance in improving the mass and heart-disease patients' cognition, understanding and trusting exercises' role in cardiac protection, push the combination of the basic research and clinic application, etc. Exercise directly reduces the risk of heart disease, improves heart damage, and optimizes cardiac metabolism and function. At the same time, exercise can indirectly protect the heart through metabolites of the intestinal flora and "exerkines" from the skeletal muscle, kidney, liver, and adipose tissue. Based on the comprehensive neural regulatory network, exercise can improve cardiac function by regulating cardiac autonomic nerve activity through the "brain-heart" axis, "gut-brain" axis and "liver-brain" axis, and optimize cardiovascular reflexes. On this basis, "exercise simulators", experimental animal models, telerehabilitation safety, efficient and accurate exercise guidelines, etc., are the opportunities and challenges faced by cardiac exercise rehabilitation when the basic research will be transferred into clinic practice. Notably, the advancement of neurocardiology is of paramount importance in elucidating the mechanism of "synchronous rehabilitation for brain and heart" in exercise.
-
Keywords:
- heart /
- exercise /
- exerkines /
- organ crosstalk /
- integrative physiology
-
随着全球老龄化进程的加速和不良生活习惯的蔓延,心血管疾病(Cardiovascular Disease,CVD)的发病率和死亡率不断攀升。截至2021年,全球CVD死亡人数已超过1 990万人,其中缺血性心脏病达920万人,位居首位,占全球CVD死亡总数的46%,相较于1990年增长了72%[1]。同样,我国城乡居民CVD死亡人数亦居于全因死亡人数的首位,农村占48.9%,城市占47.35%,其中冠心病是致死首因[2],有效防控CVD迫在眉睫。
运动是代谢性CVD的有效防控手段。临床证据表明,规律适当的体育锻炼可有效降低CVD发病率和死亡率[3]。在生理机制层面:高峰团队[4]发现,长期运动通过促进骨骼肌、肝脏等组织器官分泌miR-342-5p,经循环作用于心脏,抑制心肌细胞过度凋亡,改善心肌缺血再灌损伤;肖俊杰团队[5−7]发现,运动通过miR-210促进心肌细胞增殖及存活,提高B细胞活化阈值,增强心脏抗炎能力,并通过心肌乳酰化修饰,抑制METTL14,降低心肌mRNA m6A水平,改善心脏缺血性损伤;笔者团队[8]发现,运动通过改善大脑初级运动皮层M1区神经元损伤,抑制心交感神经恶性重构,改善心梗小鼠的心功能。可见,运动保护心脏的机制研究已经逐渐从心脏自身视角拓展到远隔器官、免疫调控和中枢调控等方面。然而,世界卫生组织的报告指出,超过1/4的成年人未能达到推荐的身体活动量(即每天150~300 min中等强度运动或75~150 min高强度运动)[9],表明运动在保护心血管方面的潜在效益未得到足够重视[10]。因此,加强代谢性疾病的运动干预及CVD的运动康复教育普及工作刻不容缓。
早在1977年首次提出的整合生理学概念及其研究方法[11]就主张将生命现象简化为微观层面的物理理论,并尝试运用物理学等非生命系统的研究方法来探究复杂的生命系统,在此基础上,2011年提出的“整合生理学2.0”方法将系统生物学的大规模无偏“组学”研究与整合生理学相结合[12],为心血管稳态的解释提供了新思路。随着组学和神经科学等新技术的不断发展,运动生理学领域迎来了新的发展机遇。这些技术不仅为揭示人体重要器官及整体功能如何从运动中获益提供了新思路,也为运动生理学研究注入了新活力。基于整合生理学视角,从心脏自身、远隔器官和中枢调控等方面,系统梳理心脏从运动中获益的文献,探讨其机制,对促进大众尤其是心脏病患者认识、理解和信任运动对心脏的保护作用,推动基础研究与临床应用的融合,具有重要的理论和现实意义。
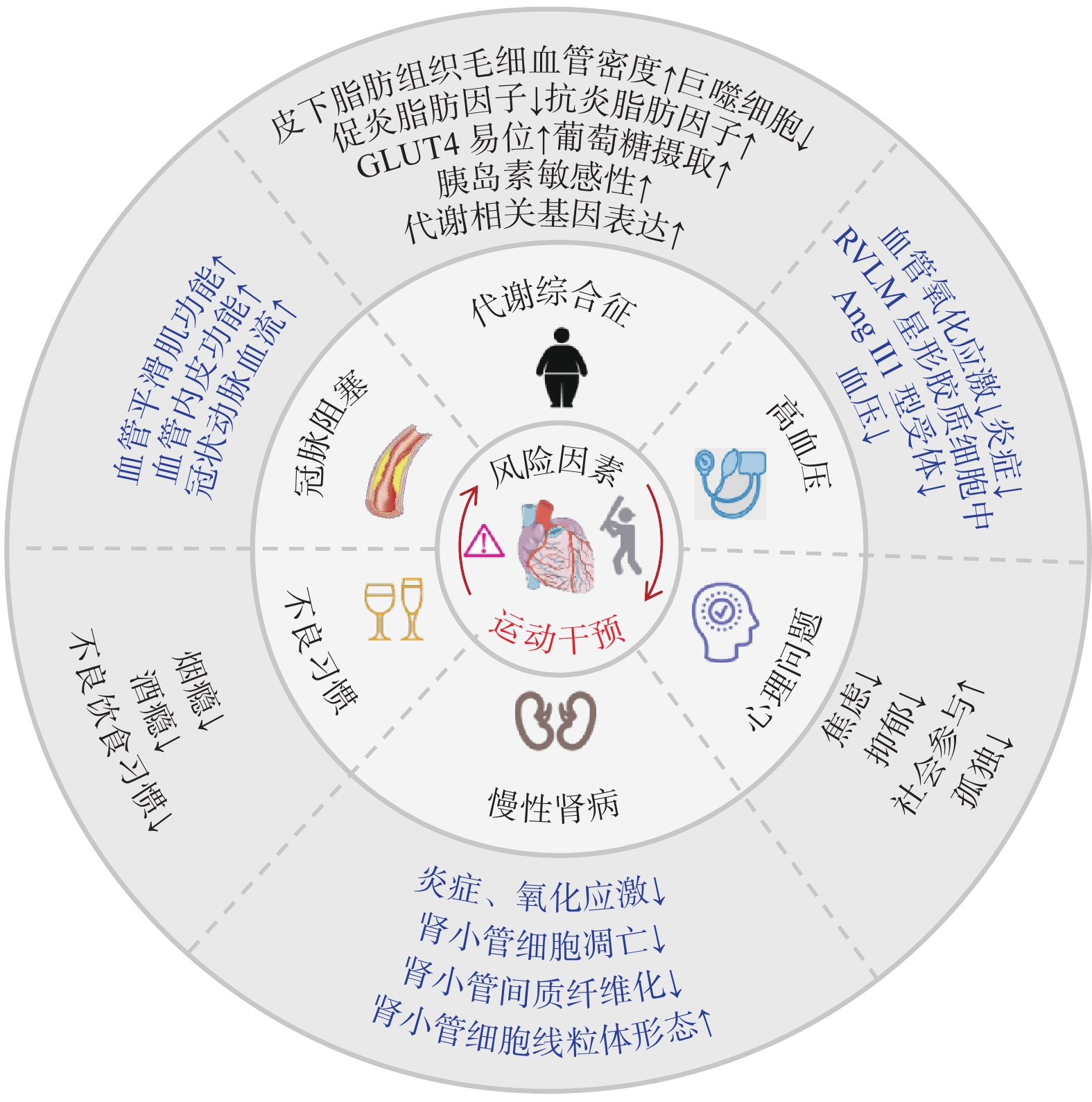
1. 运动是降低心脏健康风险的良方
1.1 影响心脏健康的主要风险因素
心脏健康风险因素包括先天性和后天性两个方面:前者通常受遗传因素影响,可导致胎儿期心脏发育异常;后者包括内源性和外源性风险因素。内源性因素包括高血压、慢性肾病、肥胖、代谢综合征和糖尿病等,其中高血压是主要致病因素之一[1]。长期高血压可引发心肌炎症、氧化应激、细胞凋亡和纤维化等病理变化,导致左心室病理性肥大以及心衰。外源性风险因素主要是不良生活习惯。在我国居民因缺血性心脏病死亡的病例中,16.38%的患者与高盐饮食有关,这一数据显著高于其他国家[13]。高脂饮食可诱发冠状动脉粥样硬化,是显著增加冠心病的重要风险因素。心理问题是极易被忽略的风险因素。焦虑、抑郁和孤独症等均可通过下丘脑-垂体-肾上腺轴触发缺血性心脏病的发生[14]。综上,后天性心脏病的风险因素众多,病因复杂。目前仍然存在许多超越传统的心脏健康威胁因素,如空气污染、声和光污染、化学暴露以及微塑料污染等,未来从暴露组学方面可进一步探究心脏疾病的风险因素,以拓展心脏疾病预防的范围。
1.2 运动可显著降低罹患心脏病的风险
循证医学证据表明,运动能够有效控制高血压的发生与发展[15]。运动可通过促进间质液流动增加切应力降低延髓腹外侧核(Rostral Ventrolateral Medulla,RVLM)星形胶质细胞的血管紧张素Ⅱ(Angiotensin Ⅱ,Ang Ⅱ)1型受体表达,抑制氧化应激和炎症反应,降低血压[16]。并且,运动可激活核呼吸因子2(Nuclear Respiratory Factor 2,Nrf2)通路,减少单核细胞浸润,有效减轻肾脏炎症和氧化应激损伤,促进一氧化氮(NO)释放,提高NO生物利用度,降低心肌Ang Ⅱ水平,抑制慢性肾病患者的心肌纤维化和左心室病理性重塑[17]。此外,运动通过调节脂肪促炎及抗炎细胞因子的平衡[18],降低巨噬细胞炎症反应[19],促进葡萄糖转运蛋白4(Glucose Transporter 4,GLUT4)易位和葡萄糖摄取,增强胰岛素敏感性,改善糖代谢异常[20],促进代谢相关基因表达[21],恢复代谢稳态,改善代谢综合征;改善血管内皮功能和血管平滑肌细胞活性,增加心外膜冠状动脉直径和毛细血管密度[22],降低代谢性心脏病和冠心病风险。对于不良习惯,运动能够降低烟瘾和酒瘾[23−24],进而降低CVD风险。此外,运动可通过调节相关脑网络、调整情绪、体验回避、提高自我效能以及奖励机制,改善抑郁和焦虑等心理问题[25],多人运动也能增加社会参与感,缓解孤独心理,降低心脏疾病风险。总之,运动作为一种非药物干预手段,对防控心脏疾病具有重要临床意义(图1)。
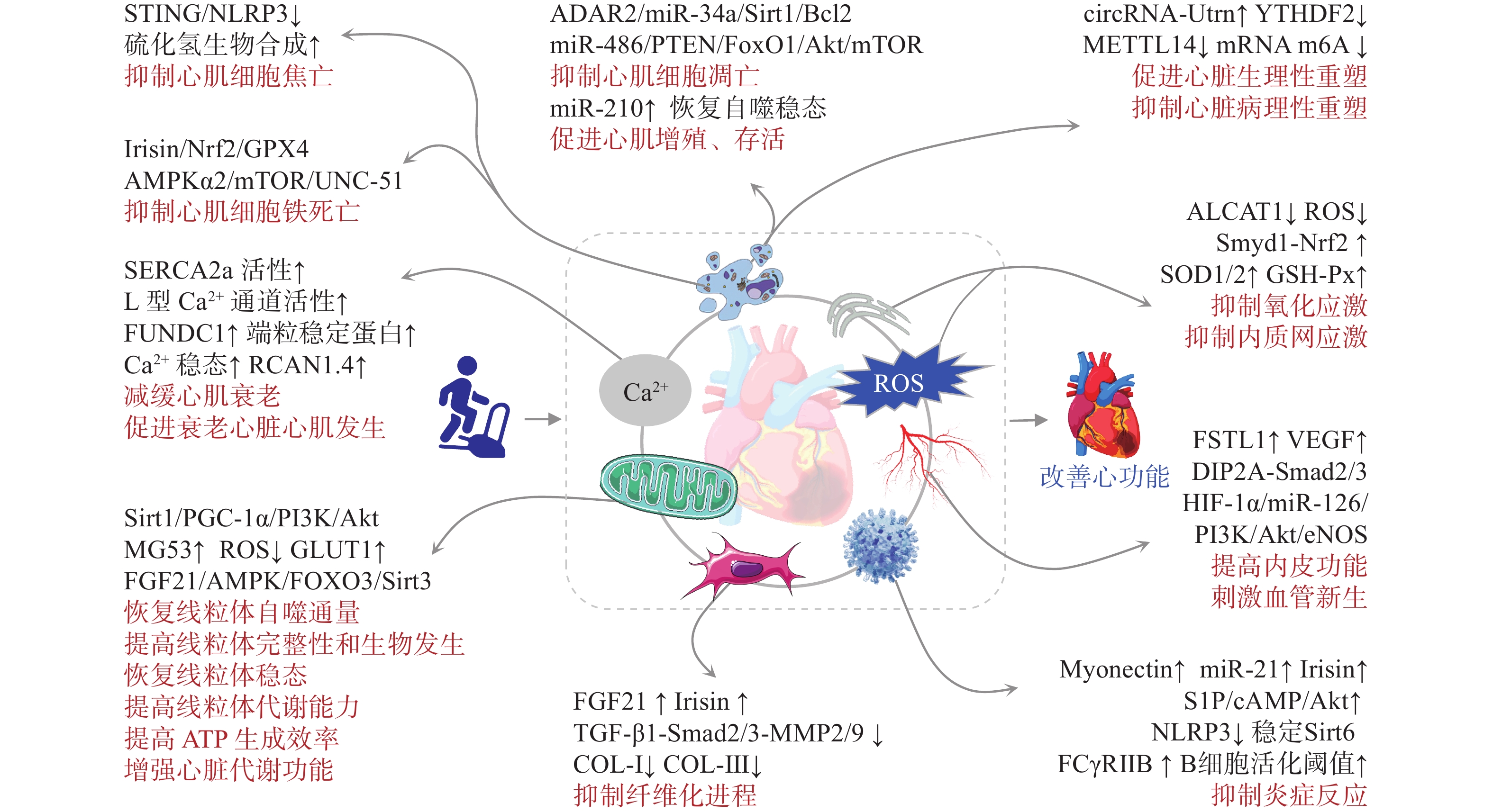
2. 运动促进心脏健康的生物学机制
2.1 运动改善心脏结构及代谢
2.1.1 运动改善细胞损伤
运动可显著改善氧化应激与内质网应激损伤。氧化应激是导致心肌细胞损伤的关键环节。研究[26−27]发现,心磷脂酰基转移酶1(Lysocardiolipin Acyltransferase 1,ALCAT1)通过催化心磷脂(Cardiolipins,CL)的病理重塑引起线粒体功能障碍,导致活性氧(Reactive Oxygen Species,ROS)爆发,而运动可显著抑制ALCAT1的表达,提高心肌超氧化物歧化酶1(Superoxide Dismutase 1,SOD1)、SOD2、谷胱甘肽过氧化物酶以及过氧化氢酶等抗氧化酶的表达和活性,降低ROS水平,并通过抑制钙/钙调蛋白依赖性蛋白激酶Ⅱ-线粒体动力蛋白相关蛋白1(Dynamin-relatedprotein 1,Drp1)的磷酸化,阻止ROS诱导的线粒体损伤,进而逆转脂质过氧化,减轻心肌氧化应激损伤。采用ALCAT1小分子抑制剂Dafa可减轻心梗后的心肌损伤和病理性重塑[27],推测ALCAT1是保护心脏的潜在“运动模拟物”。氧化应激可触发内质网应激。运动可激活中年小鼠Smyd1-Nrf2信号,引起Nrf2与Kelch样ECH相关蛋白1(Kelch Like ECH Associated Protein 1,Keap1)分离后易位至细胞核,在调控氧化基因表达的同时抑制未折叠蛋白反应,缓解心肌内质网应激,进而保护心肌细胞[28]。从胚胎期到成年期(6月龄),小鼠心脏Smyd1表达逐渐增加,到达中年期(14月龄)后表达降低[28−29]。运动可显著提高中年小鼠心肌Smyd1表达[28],推测该作用在延缓心肌衰老方面具有积极意义。
运动可改善线粒体的损伤及其功能。心肌缺血缺氧或压力超负荷均可导致线粒体结构与功能紊乱,加剧心肌细胞氧化应激损伤[30]。运动可通过磷脂酰肌醇3-激酶(Phosphoinositide 3-Kinase,PI3K)/蛋白激酶B(Protein Kinase B,Akt)激活AD-依赖性去乙酰化酶(Sirtuin-1,Sirt1)/过氧化物酶体增殖物激活受体γ-辅激活因子1-α(Peroxisome Proliferators-activated Receptor γ Coactivator-1α,PGC-1α)通路,恢复心肌线粒体功能和生物发生,调控细胞代谢,降低ROS积累和线粒体功能损伤的恶性循环,恢复线粒体稳态[31]。运动还可通过骨骼肌分泌肌因子MG53,并与心肌线粒体特异性CL结合,维持线粒体膜电位稳定性,降低线粒体ROS渗漏,减少E3泛素连接酶Parkin的募集,进而抑制线粒体自噬,发挥心肌保护作用[32]。2024年的一项研究[33]显示,心肌Uqcrfs1(线粒体呼吸链复合物Ⅲ)敲除后可降低心肌线粒体功能,提高葡萄糖利用率,恢复心肌细胞的有丝分裂能力,从而产生新的心肌细胞迁移至梗死区,保护心脏。提示,抑制心肌线粒体的氧化磷酸化可促使心肌更多地依赖葡萄糖和糖酵解途径产能(类似胎儿时期的代谢特点),这对于心肌梗死修复至关重要。运动如何调控线粒体及心肌细胞代谢促进心肌增殖,值得关注。多组学研究[34]发现,相对于其他组织器官,心肌线粒体对运动反应最敏感,但存在时间和性别差异,表明运动调节线粒体功能和代谢是保护心肌细胞的重要途径,未来应进一步关注运动参数和性别因素的影响。
运动可恢复心肌钙稳态并缓解衰老性变化。心肌细胞钙离子(Ca2+)稳态失衡可导致电生理紊乱,诱发多种代谢性心脏疾病[35]。研究[36−37]发现:主动脉狭窄可导致心衰小鼠心肌肌浆网/内质网Ca2+ ATPase 2a(Sarcoplasmic/endoplasmic Reticulum Ca2+ ATPase 2a,SERCA2a)、钠钙交换体(NCX)表达增加和L型Ca2+通道功能恶化,Ca2+稳态失衡;而运动可降低心衰心肌细胞SERCA2a和NCX表达,恢复L型Ca2+通道功能,维持心肌Ca2+稳态,改善心肌细胞舒/缩功能。此外,运动也可维持或提高心肌SERCA2a表达,其“矛盾”原因在于运动可提高SERCA2a和NCX的活性、肌丝对Ca2+的敏感性以及心肌的Ca2+处理效能,逆转SERCA2a等钙调蛋白的代偿性增加。提示,运动对心肌细胞的保护可能存在从弥补功能蛋白水平增加到功能蛋白效能提高的转变。衰老心肌细胞通常伴随多种损伤变性,运动通过过氧化物酶体增殖物激活受体γ(Peroxisome Proliferators-activated Receptors γ,PPAR γ)依赖性方式上调X三体综合征相关蛋白,抑制内皮细胞线粒体损伤并恢复线粒体自噬水平,抑制冠状动脉内皮老化;通过胰岛素样生长因子1(Insulin-like Growth Factor 1,IGF-1)和内皮型一氧化氮合酶(Endothelial Nitric Oxide Synthase,eNOS)介导,上调端粒稳定蛋白端粒重复序列结合因子2的表达,增强心肌细胞端粒的稳定性,延缓细胞衰老;通过诱导衰老心肌昼夜节律基因RCAN1.4表达,降低老年人心脏钙调磷酸酶活性,延缓心肌细胞衰老性变化[38−40]。提示,运动与生物钟相结合的综合干预可能对心脏或其他慢性疾病产生更积极的效应。另有文献[41]分析表明,通过不同方法抑制衰老成纤维细胞生成,会加重心脏纤维化。未来研究应进一步关注运动对心脏各类细胞衰老的影响及其机制。
运动可抑制细胞异常死亡并促进存活。细胞凋亡在心肌衰老和受损细胞清除与更新中发挥着重要作用,但其过度激活可导致心功能降低。近年来,肖俊杰团队[42−44]发现,运动通过促进心肌miR-486表达,靶向并抑制磷酸酯酶与张力蛋白同源物(Phosphatase and Tensin Homolog,PTEN)和FOXO1以激活Akt/雷帕霉素靶蛋白(Mammalian Target of Rapamycin,mTOR)信号通路;刺激心肌特异性合成miR-222,下调心肌同源结构域相互作用蛋白激酶2,抑制P53磷酸化;通过转录因子CCAAT/增强子结合蛋白β(CCAAT/enhancer Binding Protein β,C/EBPβ)增强心肌腺苷脱氨酶2(Adenosine Deaminases Acting on RNA,ADAR2)表达,抑制miR-34a以促进Sirt1、细胞周期蛋白D1(Cyclin D1)和B淋巴细胞瘤-2基因(B-cell Lymphoma-2,Bcl-2)表达,进而降低Bax(Bcl-2 Associated X Protein)/Bcl-2比和裂解型caspase-3/caspase-3比,减轻缺血心脏的心肌细胞过度凋亡。可见,非编码RNA在心脏运动康复中扮演着极其重要的角色,但目前大多数研究集中于miRNA,circRNA和LncRNA的效应仍需进一步研究。心肌细胞内游离铁积累可诱导脂质过氧化反应和ROS剧增,引发心肌细胞铁死亡[45]。运动预处理可通过线粒体超氧化物依赖性腺苷酸激活蛋白激酶α2[Adenosine 5'-monophosphate(AMP)-activated Protein Kinase α2,AMPKα2]信号,改善能量代谢效率,调节mTOR/UNC-51样激酶1轴,激活线粒体适应性自噬并减轻铁积累,抑制氧化应激和异常脂质代谢,进而抑制心肌细胞铁死亡的发生[46]。并且,运动可通过鸢尾素(Irisin)促使Keap1/Nrf2分离并入核,促进谷胱甘肽过氧化物酶4的表达,进而减轻铁积累和脂质过氧化,抑制心肌细胞铁死亡[47−48]。心肌细胞焦亡是通过STING/NOD样受体热蛋白结构域相关蛋白3(NOD-like Receptor Thermal Protein Domain Associated Protein 3,NLRP3)介导的心肌缺血损伤期间的关键事件[49],运动可通过促进心肌硫化氢生物合成,抑制STING/NLRP3、caspase-1和白细胞介素-1β(Interleukin-1β,IL-1β)的激活与表达,进而抑制心肌细胞焦亡,保护心功能[50−51]。应激状态下自噬过度激活或激活不足均会对心脏产生不利影响[52]。运动可通过自噬双向调节,恢复自噬稳态,促进心肌存活,提高心功能[53],但其机制仍不明确。新近文献[6]报道,运动通过miR-210靶向CDK10可促进心肌细胞增殖和存活,减轻心肌缺血再灌注损伤。miR-210是一种缺氧反应性miRNA,其是否能够介导运动保护非缺血性心脏损伤,以及诱导miR-210产生所必要的运动强度和剂量值得关注。
2.1.2 运动抑制组织病理重塑
生理性心肌重塑是心脏功能增强的基础,但病理性应激可通过多个信号途径导致心脏的病理性重塑,严重降低心功能。运动可通过心肌乳酰化修饰下调m6A读码蛋白YTHDF2,以非RNA m6A依赖的方式抑制Ras-GTP酶激活蛋白SH3结构域结合蛋白1,影响心肌细胞形态变化;抑制心肌METTL14以及Phlpp2 mRNA m6A甲基化修饰,并激活Akt-S473信号,促进心肌细胞生长;上调circRNA-Utrn并以泛素-蛋白酶体依赖的方式促进丝氨酸/苏氨酸蛋白磷酸酶5的降解,从而激活丝裂原活化蛋白激酶(Mitogen-activated Protein Kinase,MAPK)/细胞外调节蛋白激酶(Extracellular Regulated Protein Kinases,ERK)信号通路;诱导长链非编码RNA FR236703(CPhar)与结合伴侣DEAD-Box家族的RNA解旋酶DDX17结合后隔离C/EBPβ来调控下游分子激活转录因子7的表达,调节心肌细胞增殖、肥大和抗凋亡基因表达,诱导心肌的生理性重塑并减轻病理性重塑[5, 54−56]。可见,表观遗传修饰在运动减轻心肌损伤并提高心功能中扮演着重要角色,但其因果关系尚不明确,且运动引起的表观遗传修饰是否能够影响子代心脏健康,仍需进一步研究。
心肌纤维化是心脏病理重塑的主要表型之一,运动可通过刺激成纤维细胞生长因子21(Fibroblast Growth Factor 21,FGF21)和Irisin表达,通过AMPK-Sirt1信号转导,抑制转化生长因子-β1(Transforming Growth Factor-β1,TGF-β1)-Smad2/3-基质金属蛋白酶2/9通路,进而抑制胶原蛋白I(Collagen I,COL-I)和COL-Ⅲ表达,减轻心肌纤维化[57−58]。微循环功能障碍是心脏病理性重塑的重要诱因,运动可通过诱导骨骼肌的卵泡抑素样蛋白1(Follistatin-like 1,FSTL1)分泌,归巢至心肌与其受体Disco相互作用蛋白2同源物A(Disco Interacting Protein 2 Homolog A,DIP2A)结合,在不受TGF-βR1的影响下直接激活Smad2/3通路,并通过上调心肌缺氧诱导因子-1α以上调mirR-126表达,激活PI3K/Akt/eNOS以及MAPK信号,促进血管内皮生长因子(Vascular Endothelial Growth Factor,VEGF)表达,进而促进心肌的血管生成[59−60]。慢性炎症是病理性心脏重塑的关键环节,运动通过上调肌连蛋白(Myonectin)激活1-磷酸鞘氨醇(Sphingosine 1-phosphate,S1P)/环磷酸腺苷(Cyclic Adenosine Monophosphate,cAMP)/Akt通路,抑制巨噬细胞活化以及促炎因子的分泌;上调B细胞表面受体FCγRⅡB的表达,提高B细胞活化阈值,抑制B细胞的PI3K/Akt信号,进而降低促炎因子分泌水平;上调运动因子Irisin激活DnaJb3/Hsp40伴侣系统,以Hsp70依赖性方式稳定Sirt6,降低心脏血管炎症[7, 61−62]。并且在幼年期进行运动干预可通过表观遗传修饰增加CRYM启动子的组蛋白H3K4me3甲基化,进而促进CRYM及其代谢产物哌啶酸的增加,后者直到老年期均可通过抑制巨噬细胞mTORC1信号,降低促炎因子的表达,提高老年时期的抗炎免疫力[63]。这从侧面印证了加强青少年运动的重要性。
2.1.3 运动改善器官代谢
心脏是代谢活动最为旺盛的器官之一,心肌细胞舒/缩功能依赖于稳定而高效的能量供应。心脏代谢重构是生理或病理性心脏重塑的关键环节,其底物随环境压力变化而变化。人类心脏代谢主要消耗脂肪酸,其活性蛋白质分解速度约为腿部肌肉的10倍,而衰竭心脏会消耗更多的酮体和乳酸,并且蛋白水解率更高[64]。心衰心肌的高能磷酸盐含量降低,磷酸肌酸和ATP比值显著下降,导致心脏供能不足,引发心功能障碍[65]。代谢信号调节蛋白FGF21是代谢性心脏病的重要调节因子。运动可诱导心肌FGF21辅助受体β-klotho的表达,通过AMPK信号促进FOXO3磷酸化,诱导线粒体去乙酰化酶Sirt3表达,促进心肌线粒体酶簇脱乙酰化以保持线粒体完整性和功能,重建线粒体裂变融合平衡和自噬通量,提高线粒体氧化磷酸化效率,恢复心肌ATP水平,改善受损心功能[66−67]。在心脏病理性重塑过程中,能量代谢底物偏好从脂肪酸氧化转变为葡萄糖氧化,产能效率降低[68]。运动可促进线粒体的中链酰基辅酶A脱氢酶和2,4-双烯酰辅酶A还原酶1表达,提高心肌脂肪酸β氧化能力,提高心脏供能效率[69]。运动通过激活AMPK信号,促进组蛋白去乙酰化酶4的磷酸化,降低肌细胞生长因子2a的抑制作用,上调GLUT1表达,促进心肌葡萄糖摄取、糖酵解、线粒体呼吸功能和ATP生成,提高心脏代谢功能[70]。当心肌代谢结构发生变化时,如表现出类似胎儿期依赖葡萄糖和糖酵解途径产能时,心肌恢复增殖能力以修复梗死区心肌组织[33],表明代谢变化与心肌细胞增殖密切相关。运动期间葡萄糖利用增加可促进小鼠心肌生长,当生长结束后这种代谢变化则消退[71],表明运动可提高心脏代谢活跃度,这种代谢变化能否促进心肌细胞增殖保护心脏,还需要更多的实验证据。此外,运动对代谢的影响是全身性的,未来应关注运动的外周和中枢代谢调节与心脏代谢的交互作用。
综上,运动对心脏的保护作用主要体现为通过改善心脏自身损伤,抑制病理性重塑,促进心脏代谢向更高效方向适应(图2)。
2.2 运动与免疫调节
近年来发现,免疫调节在维持心脏正常功能及响应心脏损伤方面具有重要作用,由此诞生了一个新的研究领域——心脏免疫学(Cardioimmunology)[72]。在心脏(包括冠状动脉)中存在多种免疫细胞,如巨噬细胞、树突状细胞、肥大细胞、单核细胞、中性粒细胞、B细胞和T细胞等。在心肌损伤的急性期,心肌的血管内皮细胞黏附分子表达增加,心肌组织中的肥大细胞、巨噬细胞等释放趋化因子,从循环中募集大量中性粒细胞和单核细胞归巢于受损心肌组织,清除死亡细胞,激活驻留在心脏中的CC趋化因子受体2阴性(CC-chemokine Receptor 2,CCR2-)巨噬细胞(M2型)并增殖,抑制炎症反应;随着损伤的发展,单核细胞继续招募并衍生大量CCR2+巨噬细胞(M1型),促炎信号被放大,肥大细胞释放促纤维化因子,导致受损组织纤维化[73−74]。此外,循环B细胞和T细胞也会被募集至损伤组织,激活适应性免疫应答,诱导心脏重塑[75]。免疫系统对运动高度敏感,在运动过程中,淋巴细胞和中性粒细胞等被动员进入循环发挥免疫调节效应[76]。运动因子Irisin与其受体整合素αVβ5结合后,可激活JAK2-STAT6信号,促进PPARγ和Nrf2的转录,诱导巨噬细胞向M2型极化[77]。抑制性受体FcγRⅡB表达较低的B细胞具有明显的炎症表型,运动能够上调FcγRⅡB表达并提高B细胞的激活阈值,抑制B细胞PI3K/Akt信号,进而降低抗体和促炎因子的分泌水平[7]。运动能够降低高血压大鼠心脏肥大细胞的密度和脱颗粒百分比,避免肥大细胞过度激活,缓解心脏炎症反应和纤维化过程[78]。心梗后心脏的超负荷会激活胸段背根神经节(TDRG)中的Piezo1,导致神经元钙内流并增加炎症因子IL-6的释放,通过轴浆运输至心肌组织,激活心脏IL-6受体和STAT3炎症通路,形成“神经-免疫-心脏”轴,加速心脏病理性重塑[79]。推测适度运动或可通过抑制Piezo1激活和心脏的病理性重塑,减轻心脏负荷,但仍需进一步验证。近年研究[80]发现,响应运动机械刺激的新型机械敏感性脂质分解因子(Reticulocalbin-2,RCN2)能够促进成骨和淋巴细胞生成,提高机体免疫力,但RCN2能否调节心脏免疫功能值得研究。
综上,运动能有效促进巨噬细胞向M2型极化,调节免疫细胞阈值,抑制免疫细胞过度激活,减少心脏受损部位的炎性细胞浸润,进而抑制病理性心脏重塑。未来应关注树突细胞和自然杀伤细胞在运动保护心脏中的作用。
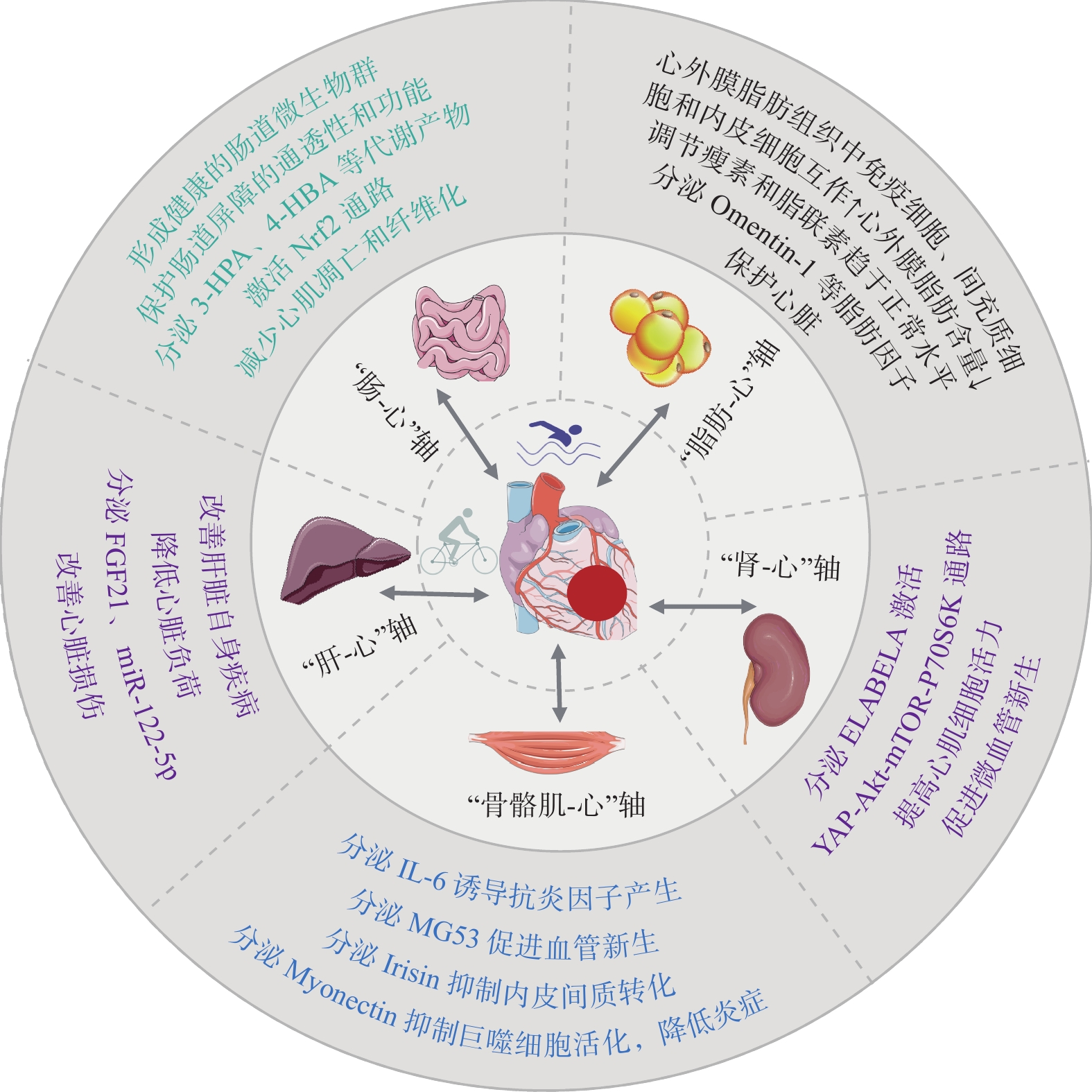
2.3 运动有助于发挥远隔组织器官的保护效应
整合生理学认为,机体各器官组织间存在广泛的交互作用。运动作为一种机械生理性刺激,远非单纯的肌肉收缩活动。运动通过刺激外周器官分泌细胞因子,如运动因子,以协调和维持机体稳态,即组织器官间的交互作用(cross talk),在心脏康复中发挥重要作用。
2.3.1 运动的“骨骼肌-心”轴作用机制
骨骼肌通过分泌多种细胞因子(或称为肌因子,Myokines)介导肌肉与多器官产生交互作用。运动诱导骨骼肌分泌IL-6经循环刺激血液单核细胞产生多种抗炎因子,或通过降低循环TNF-α水平,发挥抗炎效应[76]。研究[81]发现,脂肪来源的IL-6可促进巨噬细胞在脂肪中浸润,增加慢性炎症,而骨髓和肌肉来源的IL-6具有相反的抑制效应。因此,应关注运动过程中不同来源IL-6对心脏的差异效应。运动通过诱导骨骼肌FSTL1分泌靶向其心脏受体DIP2A,在不受TGF-βR1影响下直接激活Smad2/3通路,促进VEGF表达,进而促进心肌血管生成[59]。运动通过骨骼肌分泌肌因子MG53与心肌线粒体的CL相互作用,促进氧化应激期间线粒体膜损伤的修复,减少Parkin募集以减少溶酶体对线粒体的包裹,抑制线粒体自噬,保护心肌[32]。MG53与CL结合修复线粒体膜的机制可能类似于质膜修复,通过引入脂筏来修补受损膜。Irisin在运动后可通过促进解偶联蛋白2表达和心肌细胞自噬,抑制ROS积累,降低ROS诱导的核因子κB-Snail通路的激活,进而抑制内皮间充质转化(EndMT),改善内皮功能障碍并减轻血管周围纤维化,抑制心脏毒性损伤[82]。Myonectin在运动后通过循环到达心脏,可激活S1P/cAMP/Akt通路,抑制巨噬细胞活化和促炎因子的分泌,以及心肌细胞凋亡,保护缺血心脏[83]。但Myonectin的结合蛋白或受体目前尚不清楚。
2.3.2 运动的“肾-心”轴作用机制
心-肾综合征是临床上常见的心脏和肾脏疾病并发症。在慢性肾病状态下,高磷酸盐可下调心肌PGC-1α,导致心肌线粒体功能以及能量代谢障碍等病理性重塑[84];在急性肾病状态下,肾脏可释放IL-33并靶向诱发心肌病[85],而运动可有效抑制肾病患者的心肌损伤[17]。
运动因子通过“肾-心”轴可发挥心脏保护作用。研究[86−88]发现,由未分化的胚胎干细胞和肾脏合成的激素肽ELABELA(ELA)可增强心肌收缩力,抑制压力超负荷和Ang Ⅱ诱导的病理性心肌重塑,ELA-APJ受体轴的激活可通过降压反应、正性肌力作用、利尿、抗炎、抗纤维化和抗重塑等作用产生心血管保护效应。运动可刺激内源性ELA分泌,靶向心肌细胞通过APJ/Akt信号激活VEGF/VEGFR2和Jagged1/Notch3通路,促进心梗心肌的微血管新生,活化心肌Yes相关蛋白和核质转位,激活Akt-mTOR-P70S6K通路,促进Cyclin D1表达,提高受损心肌存活率并抑制心肌细胞凋亡,抑制病理性心肌重塑,改善心梗后的心功能[89]。ELA具有多种亚型,目前已证明Fc-ELA-21和Fc-ELA-32介导了运动对心脏的保护效应,其他ELA亚型的功能和机制有待进一步研究。
2.3.3 运动的“肠-心”轴作用机制
肠道菌群失衡不仅与胃肠道疾病密切相关,还通过多种途径直接或间接增加心脏疾病的风险[90]。运动能够通过增加氨基酸和碳水化合物代谢,优化肠道菌群组成,提高肠道微生物有益组分的丰度及多样性[91]。反之,代谢性心脏疾病可影响肠道菌群的组成和功能。有文献[92]证实,心梗后肠道微生物组分发生显著改变。因此认为,肠道与心脏之间存在的交互作用或称为“肠-心”轴。
运动可增加肠道中有益的短链脂肪酸丁酸浓度,提高有益健康菌群的比例,降低与肥胖、代谢性疾病相关的菌群(如隐性真杆菌和球状梭菌)水平,保护肠道屏障通透性和功能,降低心脏疾病的发生风险[93]。运动对肠道菌群的有益影响可不受饮食干扰,但在重返久坐生活后会消失,提示心脏病患者需要长期规律运动才能获得最大收益[93]。肠道菌群代谢产物变化也是心脏保护的重要途径[94]。2022年肖俊杰团队[95]发现,肠道菌群Lachnospiraceae_UCG-001在心梗后显著增加,Alistipes、Ruminococcus、Allobaculum和Oscillospiraceae UCG-005在心梗后显著降低。运动可显著增加心梗小鼠的Allobaculum丰度,以及菌群代谢物3-羟基吡啶甲酸(3-hydroxypicolinic Acid,3-HPA)和4-羟基丁基丙烯酸酯(4-hydroxybenzoic Acid,4-HBA)水平。通过移植运动小鼠的3-HPA和4-HBA,可激活心梗小鼠的Nrf2信号,抑制心肌细胞凋亡和纤维化反应,改善心功能,提示肠道菌群及其代谢物可作为运动诱导的潜在介导分子,开发这种运动模拟物(如有益菌)将为运动不耐受患者改善心脏代谢及康复提供新思路。
2.3.4 运动的“肝-心”轴作用机制
心脏与肝脏之间存在复杂的交互作用,如非酒精性脂肪性肝病(Nonalcoholic Fatty Liver Disease,NAFLD)患者在纤维化程度较严重时,可发生冠状动脉微血管病变和心自主神经功能障碍,加速心律失常和病理性心脏重塑,最终导致心衰[96],而运动可有效改善NAFLD[97],缓解心自主神经失衡,预防心脏的病理性变化。心脏损伤也常伴随肝损伤,而运动可通过Irisin促进肝脏巨噬细胞由M1促炎表型向M2抗炎表型极化,降低肝脏炎症反应,进而改善心功能[98]。
肝脏分泌的因子简称肝因子,研究[99]发现,运动可刺激肝脏产生miR-122-5p并通过细胞外囊泡穿梭,靶向内皮细胞,抑制酰基甘油-3-磷酸O-酰基转移酶1,进而提高脂肪酸利用和VEGF信号传导,促进微血管新生。运动可通过促进肝脏分泌运动因子FGF21,激活心肌FGF21受体,诱导线粒体脱乙酰酶Sirt3表达,促进脂肪酸氧化和ATP生成,降低ROS,缓解心肌细胞凋亡和纤维化,改善心功能受损,敲除Fgf21后这一作用被削弱[66, 100]。运动诱导的FGF21还可靶向脂肪组织,增加脂联素分泌,增强脂肪酸氧化,减轻异位脂质积累,从而增强胰岛素敏感性和代谢稳态[101]。FGF21的心脏保护作用与肝脏STAT3激活(而非心脏STAT3)直接相关,FGF21通过激活肝脏STAT3信号转导,可诱导心肌细胞编码胆固醇生物合成关键基因Acly、Lss、Sqle、Stard4和Ldlr等的表达,促进心肌细胞存活,并抑制心肌caspase-3等促凋亡基因表达,保护心梗心脏[102]。该证据进一步明确了“肝-心”轴的存在。但目前尚不清楚肝脏STAT3激活如何诱导心脏存活基因的表达,有待进一步研究。
2.3.5 运动的“脂肪-心”轴作用机制
肥胖以及代谢综合征是心脏疾病的重要诱因,肥胖引发的全身性炎症反应,通过驱动对脂肪生成尤为敏感的心外膜脂肪组织生成,导致心脏脂肪积累,诱导炎症反应,进而影响心功能[103],表明脂肪与心脏之间存在交互作用,或称为“脂肪-心”轴调节。研究[104]发现,运动通过激活心外膜脂肪组织的免疫细胞、间充质细胞和内皮细胞间的相互作用,形成抗炎微环境,减少心外膜脂肪含量。另有文献[105]报道,心外膜脂肪对心脏的影响存在双向性,射血分数降低型心力衰竭患者心外膜脂肪组织体积减少与代谢功能障碍有关,而射血分数保留型心力衰竭患者较高的心外膜脂肪组织成分与不良血流动力学特征相关,但具体机制尚未阐明。
脂肪组织分泌的瘦素和脂联素对维持心功能具有重要意义,但其水平变化异常可诱导心功能障碍[106]。运动可有效恢复超重和肥胖患者循环瘦素和脂联素的异常变化[107],其机制可能与体重和慢性炎症降低、胰岛素敏感性提高相关。新型脂肪因子Omentin-1可通过Sirt3/FOXO3a信号传导,促进心肌PINK1/Parkin依赖性线粒体自噬、线粒体融合蛋白2和视神经萎缩因子1的表达,减少线粒体Drp1积累,维持线粒体融合/分裂平衡,减小心肌梗死面积,改善心肌缺血性损伤[108]。临床研究[109]显示,运动可增加血清Omentin-1含量,提示运动可能通过Omentin-1保护受损心脏,但其因果关系与机制需要进一步探索。脂肪动员需要一定的运动强度和剂量,未来应关注不同运动方案与脂肪因子分泌对心脏保护效应的量效关系。
综上,运动通过骨骼肌、肾、肠、肝等器官以及脂肪组织的远隔组织器官交互作用,抑制炎症、调节脂肪因子、改善线粒体功能和促进血管新生等,对提高心脏健康和降低心脏疾病风险具有重要意义(图3)。未来研究应进一步挖掘各器官组织有益于心脏的运动因子,为心脏康复提供更多的潜在靶点。
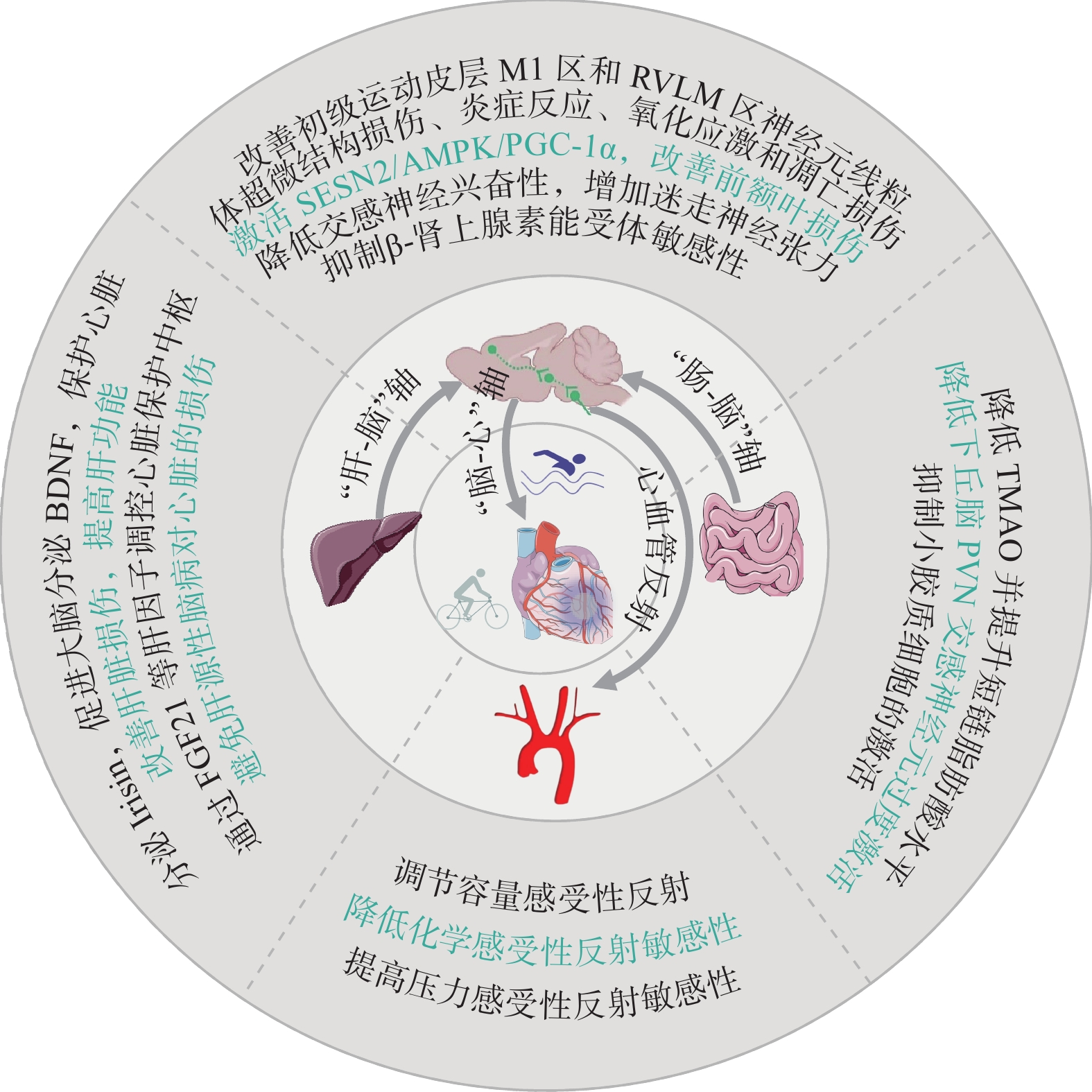
2.4 运动通过中枢对外周器官的调控作用
脑科学是运动科学的重点交叉领域,近年发现,运动通过中枢调控和心血管反射发挥心脏保护效应,强调了“脑-心”轴的重要联系。同时“远隔器官-脑-心”之间的多器官交互也不断被证实。区别于“远隔器官-心”轴,如“肠-心”和“肝-心”等轴之间的直接交互,“远隔器官-脑-心”轴体现了中枢对外周器官如肠道、肝与心之间的调控作用,其调控机制涉及心交感和迷走神经的反馈回路和多重信号。运动可通过直接或间接途径改善大脑相关损伤或功能,调节心自主神经功能,发挥心脏保护效应。
2.4.1 运动的“脑-心”轴作用机制
心交感神经与迷走神经失衡是导致心衰的重要原因之一。心肌受损或心脏负荷增加时,交感神经活动增强,去甲肾上腺素释放增加和再摄取减少,引起左心室收缩增强和心率加快,该机制的过度激活会加重心肌损伤和病理性重构,加速心衰,形成恶性循环。迷走神经的适当激活可改善左心室血流动力学,增加心率变异性(Heart Rate Variability,HRV),改善迷走神经反射,增加NO释放,改善心功能[110]。运动可通过减少交感神经过度激活,增加迷走神经张力,改善HRV和肾上腺素能受体敏感性,降低室性心律失常的风险[111]。该途径是运动通过自主神经调节保护心脏的经典途径,但运动对心自主神经影响的中枢机制尚未完全阐明。
心功能的维持取决于外周和中枢神经系统之间持续复杂的通信。有文献[112]报道:左心室注射PRV Bartha病毒5~6 d后在初级运动皮层M1区发现了荧光标记,提示初级运动皮层M1区与心脏存在解剖学联系;采用光遗传激活或抑制M1区谷氨酸能神经元活动可调控正常小鼠和心梗小鼠的心功能和血压。通过跨突触顺行追踪技术,发现M1下游的中缝正中核(MnR)是调控正常和心梗小鼠心功能和血压的中续核团[113],这是“脑-心”轴调节心功能的新途径。进一步研究[8]发现,心梗心脏的初级运动皮层M1区L5层神经元出现线粒体肿胀、分裂和嵴溶解等结构损伤,神经元发生凋亡和炎症反应,心交感神经张力增加,而运动可逆转该神经元损伤,进而减轻心交感神经重构,提高心梗小鼠的心功能。心衰小鼠RVLM脑区Nrf2、NAD(P)H醌脱氢酶 1[NAD(P)H Dehydrogenase Quinone 1,NQO-1]和血红素氧合酶1表达显著降低,Nrf2信号传导和抗氧化防御系统受损,导致交感神经兴奋性增加,进而破坏外周器官功能并加剧心衰,在RVLM中选择性过表达Nrf2则可逆转上述病理过程[114]。运动干预可显著上调心衰小鼠RVLM脑区Nrf2和抗氧化酶NQO-1表达,减少去甲肾上腺素释放,降低交感神经兴奋性,保护心衰心脏[115]。心脏的机械压力超负荷时,交感神经传出末梢释放的ATP可通过刺激P2X7受体,激活心脏非免疫细胞中的NLRP3炎性小体,引起炎症反应和心肌肥大[116]。但运动能否通过调控交感神经末梢递质释放影响心脏结构和功能损伤值得探究。此外,有氧运动通过减少氧化应激、神经胶质细胞活化和神经炎症,可减轻脂多糖诱导的认知功能障碍[117];通过激活应激诱导蛋白2/AMPK/PGC-1α通路,可抑制神经元氧化应激和炎症,减轻心梗小鼠前额叶损伤和心功能障碍[118],表明运动通过“脑-心”轴调控自主神经,对心脏产生保护作用。
2.4.2 运动的心血管反射调控机制
心脏活动受心血管反射的精细调节,包括压力和化学感受性反射以及容量感受性反射,其敏感性在慢性心衰中具有重要的预后价值[119]。在高血压患者中普遍存在压力感受性反射障碍,而运动结合健康饮食及体重管理可有效改善顽固性高血压患者压力反射的敏感性,提高心率变异性[120],减轻心脏负荷,改善心脏疾病预后,其机制可能涉及运动导致血管弹性增加以改善压力感受性反射的机械转导信号,提高迷走神经兴奋性,以及增加心脏胆碱能反应性。有20%的心衰患者的化学感受性反射异常敏感,并表现出较高的肾上腺素能激活和中枢性呼吸暂停风险[119]。有研究[121]发现,心衰小鼠脑干后梯形后核(Retrotrapezoid Nucleus,RTN)发生氧化应激反应并存在化学感受性反射的敏感性增加和呼吸障碍,而运动训练可抑制RTN区ROS水平并提高抗氧化基因表达,促使化学感受性反射的敏感性正常化,消除心衰小鼠的呼吸障碍,改善心衰预后。此外,心功能障碍尤其是心衰通常伴随容量超负荷[122],容量感受性反射被加强,导致心输出量降低。运动可通过促进血液重新再分配到运动系统,缓解循环容量超负荷,进而降低容量感受性反射,改善心功能,但运动对心血管反射敏感性调控的中枢机制并未完全阐明。
2.4.3 运动的“肠-脑-心”轴作用机制
“肠-脑”轴参与心脏疾病的发生发展。肠道微生物衍生的代谢物氧化三甲胺(Trimethylamine N-oxide,TMAO)可激活下丘脑视旁核(PVN)的交感神经核,促进左星状神经节(LSG)部位的兴奋性受体NMDAR表达,直接激活LSG部位的神经元,并促进该区的促炎标志物IL-1β、IL-6和TNF-α表达,引起炎症反应,激活心交感神经,发生缺血后的室性心律失常,缩短心房有效不应期并增加心房易损窗,进而促使房颤发展[123]。运动结合低热量饮食可显著降低肥胖成年女性的血浆TMAO水平[124],进而避免TMAO激活LSG诱导的心交感神经激活,降低心脏负荷和心律失常的发生,其机制可能是运动通过影响肠道菌群的组成和活性,改善机体的代谢状态,减少食物中TMAO前体(如胆碱和左旋肉碱)在肠道中的积累,或通过抑制肝脏黄素单加氧酶(Flavin-containing Monooxy Genases,FMO1)和FMO3(催化产生TMAO的主要酶类)表达,抑制TMAO产生。此外,运动可降低肠道菌群失调标志物厚壁菌门与拟杆菌门的比值,增加产生乙酸盐的菌群并减少产生乳酸的菌群,抑制回肠中TNF-α和IL-6等促炎基因的表达,改善回肠通透性,进而抑制PVN区小胶质细胞的激活和炎症反应,降低血压和心脏压力超负荷损伤[125]。因此,肠道菌群及其代谢产物可通过中枢影响心功能,而运动可通过“肠-脑”轴调控心自主神经系统活动,降低心脏负荷,产生心脏保护作用。
2.4.4 运动的“肝-脑-心”轴作用机制
多器官影像学研究[126]发现,肝、脑和心之间存在丰富的交互作用,推测“肝-脑-心”存在相互调节作用。研究[127]发现,NAFLD诱导的全身性炎症和内皮功能障碍与动脉硬化有关,并显著增加缺血性脑中风的发生风险。脑缺血后早期的区域神经炎症可导致心功能不全[128]。衰老肝脏产生的可溶性环氧化物水解酶可破坏14,15-环氧二十碳三烯酸和脑β淀粉样蛋白的平衡,诱导阿尔茨海默病的发生,增加心衰风险[129−130]。运动通过改善肝功能[97],可预防并改善肝性脑损伤引起的炎症交互和其他神经性疾病,发挥心脏保护作用。通过对肝脏过表达FNDC5分泌Irisin经循环可靶向作用于海马体,促进海马体脑源性神经营养因子(Brain-derived Neurotrophic Factor,BDNF)表达,并与原肌球蛋白受体激酶B结合,激活下游ERK1/2和Akt通路,促进心脏驻留巨噬细胞M2抗炎表型的存活,抑制炎症反应并分泌VEGF促进血管新生,抑制心肌细胞凋亡和纤维化,修复梗死心脏[131−132]。因此推测,运动通过Irisin-BDNF介导对“肝-脑”轴产生影响是保护心脏的途径之一。肝源性运动因子FGF21可直接激活蓝斑(LC)的去甲肾上腺素能神经元[133],激活中脑腹侧被盖区VTA-LC回路可调节肝功能,促进补体C3的增加,促进血管新生,抑制炎症反应和纤维化,有助于修复梗死心脏[134]。
综上,运动可通过“心-脑”轴、心血管反射、“肠-脑-心”轴和“肝-脑-心”轴等多轴交互,减轻脑损伤,恢复心自主神经平衡发挥脑-心双向保护作用(图4),但其机制有待进一步阐明。“2024年首届神经心脏病学会议”对“脑心共患病”进行了热议,关注神经心脏病学发展和“脑心同治”“脑心共研”“脑心同康”,对揭示运动的“脑心同康”机制具有重要意义。
3. 运动保护心脏:从基础到临床的机遇与挑战
3.1 “运动因子”和动物实验成果的临床转化研究
3.1.1 “运动模拟物”在运动受限患者心脏康复中的潜在价值
近年来,在运动科学和康复医学领域,“运动因子”受到广泛关注。在运动与心脏保护研究领域已发现诸多“运动因子”,通过不同的机制和途径参与心脏保护(表1)。随着转化医学的推进,“运动因子”及其靶标为开发模拟运动效应的潜在“运动模拟物”提供了新思路。SCENT(Stem Cell-derived Exosome Nebulization Therapy)作为一种无创的外泌体递送方法,通过雾化吸入方式靶向递送,对心梗的治疗具有显著效果[135]。该方法为运动受限患者的心脏康复提供了新的治疗策略。
表 1 参与心脏保护的运动因子及其作用Table 1. Exerkines involved in cardioprotection and their roles运动因子 生理作用 参考文献 FGF21 抑制纤维化、氧化应激和细胞凋亡 [57] IGF-1 抑制心肌纤维化 [136] CCDC80tide 抑制纤维化和病理性重塑 [137] CPhar 抑制纤维化、细胞凋亡、促进生理性肥大 [56] MG53 维持线粒体完整性,抑制氧化应激 [32] IL-10 增加髓源性抑制细胞,抑制炎症反应 [138] Myonectin 抑制细胞凋亡和炎症反应 [62] Irisin 抑制纤维化,增强线粒体功能 [58, 139] Mhrt779 维持生理性肥大记忆 [140] Circ-Ddx60 维持生理性肥大记忆 [141] Circ-Utrn 促进心肌存活,抑制细胞凋亡和纤维化 [54] ELABELA 维持心肌活力,促进血管新生 [89] miR-486 抑制纤维化和细胞凋亡 [43] miR-342-5p 提高心肌活力,抑制细胞凋亡 [4] ADAR2 促进增殖,抑制细胞凋亡 [42] miR-17-3p 促进心肌增殖、存活 [142] miR-210 促进心肌增殖、存活 [6] miR-126 促进血管新生 [60] miR-122-5p 促进血管新生 [99] FSTL1 促进血管新生 [59] 3.1.2 模拟临床疾病及其运动干预的实验动物模型有待完善
研究模型制备是基础研究中验证科学假设最重要的求证范式。在心脏运动康复领域,常用动物模型涵盖斑马鱼、小鼠及大鼠。小鼠和大鼠能经冠状动脉结扎或栓塞制备心梗、心肌缺血再灌注模型,高脂与药物诱导则被用于冠状动脉粥样硬化模型。病理性心肌肥厚模型通过肾动脉狭窄、Ang Ⅱ诱导、高盐饮食或遗传学手段(如自发性高血压模型)制备。射血分数降低型心衰由冠状动脉结扎或阿霉素诱导,射血分数保留型心衰采用高血压、肥胖、压力超负荷和衰老模型。斑马鱼基因突变、心脏手术局部冷冻及心尖切除等方法可构建先天性心脏缺陷、心肌损伤与修复模型。动物运动干预的常见方法包括跑台模拟有氧运动、间歇运动、高强度间歇训练(High-intensity Interval Training,HIIT)及负重爬梯模拟抗阻运动。在临床心脏康复中,多样化运动疗法(如太极拳、韵律体操、骑行、神经肌肉刺激等)疗效显著。细胞水平则采用切应力、拉应力、压应力等应力培养模型。但如何准确模拟动物运动干预与细胞实验中的量效关系,以获取贴近临床的表型并揭示分子机制,仍是挑战。未来研究需聚焦贴近临床的干预与模拟方案,为临床研究及转化应用提供坚实数据支撑。
在动物模型中,需谨慎考虑物种差异。基础研究需严格控制基线参数(性别、年龄、疾病状态、饮食习惯等)以减少干扰。在临床心脏康复中,患者个体差异大,如何运用基础研究与临床大数据模拟,有效甄别这些差异,是当前研究的重要课题。新近文献[34, 143−144]报道,体育活动分子传感器联盟(MoTrPAC)采集不同性别动物、不同时间段耐力训练后的不同组织器官和线粒体对运动训练反应的多组分、多组织时间图谱以及人的骨骼肌、脂肪和血液等数据,利用全生物组和多组学方法构建了运动响应的分子图谱,结果表明运动可降低疾病风险,对心脏产生有益效应,且覆盖诸多组织、器官和系统以及跨系统的细胞和分子适应。采用多组学(包括表观基因组、单细胞组、转录组、蛋白质组、代谢组和空间表型组)全面揭示不同时间段及生物节律特征、不同性别、不同器官组织,响应不同运动方式和运动强度产生的心脏保护效益,是未来研究的重点。
3.2 心脏运动康复的远程数字化研究
远程数字化心脏康复方法可借助可穿戴设备、远程监测和AI技术为患者提供便捷的康复途径。当前需明确运动强度、时间与潜在“运动因子”的量效关系,以优化居家康复方案,科学跟踪患者的运动量、心率等指标。有文献[145]报道,居家远程心脏运动康复的效果与住院或在康复中心接受的康复治疗并无显著差异。这种居家远程康复方式具有较高便利性,但针对运动不耐受和高危人群,提前预测患者的运动风险和快速援助响应显得尤为重要。在基础和临床研究领域,深入挖掘心血管事件的预测生理指标,对居家心脏运动康复的未来方向具有决定性作用。
3.3 组合型运动辅助疗法与个性化精准运动处方
(1)组合型运动辅助疗法。心脏运动康复是一个综合干预过程,涵盖运动、药物、营养、心理及生活方式调整,联合应用这些措施对提升疗效至关重要。研究[146]显示,运动联合二甲双胍的干预效果优于单一治疗。因此,跨学科合作探索组合疗法,例如饮食和心理等联合干预,可为患者提供更全面的康复指导,是心脏康复领域的重要发展方向。
(2)个性化精准运动处方。依据FITT-VP原则,制定心脏病患者精准运动处方需考虑运动频度(Frequency)、强度(Intensity)、时长(Time)、类型(Type)、总量(Volume)和进阶(Progression)。个性化处方对心脏康复至关重要。研究[147−148]显示,心衰患者心肺健康随运动强度增加而提升,联合有氧、抗阻、吸气肌训练效果更佳。HIIT对冠心病预后效果优于其他运动[149],但风险也更高。有氧和抗阻训练可减少心外膜脂肪,仅抗阻训练可减少心包脂肪[150]。未来需研发多样化、个性化的精准运动处方,指导临床康复实践。
综上,心脏运动康复需个性化考虑患者差异,制定科学有效的运动处方。随着临床应用的发展,制定代谢性心脏疾病运动指南至关重要。
4. 结束语
运动能够有效降低罹患心脏疾病的风险,改善心脏自身结构损伤,提高心脏代谢能力,改善心功能障碍。运动介导远隔组织器官如骨骼肌、肾、肠、肝、脂肪等与心脏的交互作用,通过“脑-心”轴、心血管反射、“肠-脑-心”轴和“肝-脑-心”轴等多轴机制,恢复心自主神经平衡,发挥心脏保护作用。关注神经心脏病学发展和“脑心同治”“脑心共研”“脑心同康”,对揭示运动的“脑心同康”机制具有重要意义。在基础-临床转化研究中,“运动因子”及其“运动模拟物”有待进一步挖掘开发,实验动物模型有待完善,心脏远程康复的安全性亟待加强,应重视组合型疗法和个性化运动处方的研制。随着运动与心脏保护机制的深入研究与临床应用的推广,应重视代谢性心脏疾病的运动康复专家共识和指南的研制。
作者贡献声明:田振军:提出论文主题,设计、优化论文框架,撰写、修改论文;作者贡献声明:王涛:收集、梳理文献,绘制图表,撰写论文。 -
表 1 参与心脏保护的运动因子及其作用
Table 1 Exerkines involved in cardioprotection and their roles
运动因子 生理作用 参考文献 FGF21 抑制纤维化、氧化应激和细胞凋亡 [57] IGF-1 抑制心肌纤维化 [136] CCDC80tide 抑制纤维化和病理性重塑 [137] CPhar 抑制纤维化、细胞凋亡、促进生理性肥大 [56] MG53 维持线粒体完整性,抑制氧化应激 [32] IL-10 增加髓源性抑制细胞,抑制炎症反应 [138] Myonectin 抑制细胞凋亡和炎症反应 [62] Irisin 抑制纤维化,增强线粒体功能 [58, 139] Mhrt779 维持生理性肥大记忆 [140] Circ-Ddx60 维持生理性肥大记忆 [141] Circ-Utrn 促进心肌存活,抑制细胞凋亡和纤维化 [54] ELABELA 维持心肌活力,促进血管新生 [89] miR-486 抑制纤维化和细胞凋亡 [43] miR-342-5p 提高心肌活力,抑制细胞凋亡 [4] ADAR2 促进增殖,抑制细胞凋亡 [42] miR-17-3p 促进心肌增殖、存活 [142] miR-210 促进心肌增殖、存活 [6] miR-126 促进血管新生 [60] miR-122-5p 促进血管新生 [99] FSTL1 促进血管新生 [59] -
[1] MARTIN S S,ADAY A W,ALMARZOOQ Z I,et al. 2024 heart disease and stroke statistics:A report of US and global data from the American Heart Association[J]. Circulation,2024,149(8):e347-e913
[2] 国家心血管病中心,中国心血管健康与疾病报告编写组,胡盛寿. 中国心血管健康与疾病报告2023概要[J]. 中国循环杂志,2024,39(7):625-660 doi: 10.3969/j.issn.1000-3614.2024.07.001 [3] TUCKER W J,FEGERS-WUSTROW I,HALLE M,et al. Exercise for primary and secondary prevention of cardiovascular disease JACC focus seminar 1/4[J]. Journal of the American College of Cardiology,2022,80(11):1091-1106 doi: 10.1016/j.jacc.2022.07.004
[4] HOU Z X,QIN X H,HU Y Y,et al. Longterm exercise-derived exosomal miR-342-5p:A novel exerkine for cardioprotection[J]. Circulation Research,2019,124(9):1386-1400
[5] XU G E,YU P J,HU Y X,et al. Exercise training decreases lactylation and prevents myocardial ischemia–reperfusion injury by inhibiting YTHDF2[J]. Basic Research in Cardiology,2024,119(4):651-671 doi: 10.1007/s00395-024-01044-2
[6] BEI Y H,WANG H Y,LIU Y,et al. Exercise-induced miR-210 promotes cardiomyocyte proliferation and survival and mediates exercise-induced cardiac protection against ischemia/reperfusion injury[J]. Research,2024,7:0327 doi: 10.34133/research.0327
[7] WANG J,LIU S Q,MENG X X,et al. Exercise inhibits doxorubicin-induced cardiotoxicity via regulating B cells[J]. Circulation Research,2024,134(5):550-568 doi: 10.1161/CIRCRESAHA.123.323346
[8] 薄文艳,蔡梦昕,田振军. 有氧运动通过“脑-心” 轴抑制交感神经过度激活改善心肌梗死小鼠心功能[J]. 体育科学,2024,44(3):30-40 [9] BULL F C,AL-ANSARI S S,BIDDLE S,et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour[J]. British Journal of Sports Medicine,2020,54(24):1451-1462 doi: 10.1136/bjsports-2020-102955
[10] BOZKURT B,FONAROW G C,GOLDBERG L R,et al. Cardiac rehabilitation for patients with heart failure JACC expert panel[J]. Journal of the American College of Cardiology,2021,77(11):1454-1469 doi: 10.1016/j.jacc.2021.01.030
[11] IBERALL A S. A field and circuit thermodynamics for integrative physiology. I. Introduction to the general notions[J]. American Journal of Physiology-Regulatory,Integrative and Comparative Physiology,1977,233(5):R171-R180 doi: 10.1152/ajpregu.1977.233.5.R171
[12] KUSTER D W D,MERKUS D,VAN DER VELDEN J,et al. 'integrative physiology 2.0':Integration of systems biology into physiology and its application to cardiovascular homeostasis[J]. The Journal of Physiology,2011,589(5):1037-1045 doi: 10.1113/jphysiol.2010.201533
[13] WANG H,ZHANG H,ZOU Z Y. Changing profiles of cardiovascular disease and risk factors in China:A secondary analysis for the Global Burden of Disease Study 2019[J]. Chinese Medical Journal,2023,136(20):2431-2441 doi: 10.1097/CM9.0000000000002741
[14] MONTONE R A,CAMILLI M,CALVIERI C,et al. Exposome in ischaemic heart disease:Beyond traditional risk factors[J]. European Heart Journal,2024,45(6):419-438 doi: 10.1093/eurheartj/ehae001
[15] EDWARDS J J,DEENMAMODE A H P,GRIFFITHS M,et al. Exercise training and resting blood pressure:A large-scale pairwise and network meta-analysis of randomised controlled trials[J]. British Journal of Sports Medicine,2023,57(20):1317-1326 doi: 10.1136/bjsports-2022-106503
[16] MURASE S,SAKITANI N,MAEKAWA T,et al. Interstitial-fluid shear stresses induced by vertically oscillating head motion lower blood pressure in hypertensive rats and humans[J]. Nature Biomedical Engineering,2023,7(11):1350-1373 doi: 10.1038/s41551-023-01061-x
[17] BISHOP N C,BURTON J O,GRAHAM-BROWN M P M,et al. Exercise and chronic kidney disease:Potential mechanisms underlying the physiological benefits[J]. Nature Reviews Nephrology,2023,19:244-256 doi: 10.1038/s41581-022-00675-9
[18] PITA-GRISANTI V,VELEZ-BONET E,CHASSER K,et al. Physical activity decreases inflammation and delays the development of obesity-associated pancreatic ductal adenocarcinoma[J]. Cancer Research,2024,84(18):3058-3071 doi: 10.1158/0008-5472.CAN-23-1045
[19] AHN C,ZHANG T,YANG G,et al. Years of endurance exercise training remodel abdominal subcutaneous adipose tissue in adults with overweight or obesity[J]. Nature Metabolism,2024,6:1819-1836 doi: 10.1038/s42255-024-01103-x
[20] STOCKS B,ZIERATH J R. Post-translational modifications:The signals at the intersection of exercise,glucose uptake,and insulin sensitivity[J]. Endocrine Reviews,2022,43(4):654-677 doi: 10.1210/endrev/bnab038
[21] BEALS J W,KAYSER B D,SMITH G I,et al. Dietary weight loss-induced improvements in metabolic function are enhanced by exercise in people with obesity and prediabetes[J]. Nature Metabolism,2023,5:1221-1235 doi: 10.1038/s42255-023-00829-4
[22] AENGEVAEREN V L,MOSTERD A,SHARMA S,et al. Exercise and coronary atherosclerosis[J]. Circulation,2020,141(16):1338-1350 doi: 10.1161/CIRCULATIONAHA.119.044467
[23] GUNILLASDOTTER V,ANDRÉASSON S,HALLGREN M,et al. Exercise as treatment for alcohol use disorder:A qualitative study[J]. Drug and Alcohol Review,2022,41(7):1642-1652 doi: 10.1111/dar.13527
[24] ZHOU Y H,FENG W X,GUO Y G,et al. Effect of exercise intervention on smoking cessation:A meta-analysis[J]. Frontiers in Physiology,2023,14:1221898 doi: 10.3389/fphys.2023.1221898
[25] SMITH P J,MERWIN R M. The role of exercise in management of mental health disorders:An integrative review[J]. Annual Review of Medicine,2021,72:45-62 doi: 10.1146/annurev-med-060619-022943
[26] LIU N,ZHU Y N,SONG W,et al. Cardioprotection attributed to aerobic exercise-mediated inhibition of ALCAT1 and oxidative stress-induced apoptosis in MI rats[J]. Biomedicines,2022,10(9):2250 doi: 10.3390/biomedicines10092250
[27] JIA D D,ZHANG J,NIE J,et al. Cardiolipin remodeling by ALCAT1 links hypoxia to coronary artery disease by promoting mitochondrial dysfunction[J]. Molecular Therapy,2021,29(12):3498-3511 doi: 10.1016/j.ymthe.2021.06.007
[28] TANG J,WU C Y,XU Y X,et al. Resistance training up-regulates Smyd1 expression and inhibits oxidative stress and endoplasmic reticulum stress in the heart of middle-aged mice[J]. Free Radical Biology and Medicine,2024,210:304-317 doi: 10.1016/j.freeradbiomed.2023.11.029
[29] WANG Z,SCHWARTZ R J,LIU J,et al. Smyd1 orchestrates early heart development through positive and negative gene regulation[J]. Frontiers in Cell and Developmental Biology,2021,9:654682 doi: 10.3389/fcell.2021.654682
[30] HINTON A Jr,CLAYPOOL S M,NEIKIRK K,et al. Mitochondrial structure and function in human heart failure[J]. Circulation Research,2024,135(2):372-396 doi: 10.1161/CIRCRESAHA.124.323800
[31] JIA D D,HOU L,LV Y Z,et al. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1α/PI3K/Akt signaling[J]. Journal of Cellular Physiology,2019,234(12):23705-23718 doi: 10.1002/jcp.28939
[32] GUMPPER-FEDUS K,PARK K H,MA H,et al. MG53 preserves mitochondrial integrity of cardiomyocytes during ischemia reperfusion-induced oxidative stress[J]. Redox Biology,2022,54:102357 doi: 10.1016/j.redox.2022.102357
[33] WAYPA G B,SMITH K A,MUNGAI P T,et al. Mitochondria regulate proliferation in adult cardiac myocytes[J]. Journal of Clinical Investigation,2024,134(13):e165482 doi: 10.1172/JCI165482
[34] AMAR D,GAY N R,JIMENEZ-MORALES D,et al. The mitochondrial multi-omic response to exercise training across rat tissues[J]. Cell Metabolism,2024,36(6):1411-1429. e1-e10
[35] MORCIANO G,RIMESSI A,PATERGNANI S,et al. Calcium dysregulation in heart diseases:Targeting calcium channels to achieve a correct calcium homeostasis[J]. Pharmacological Research,2022,177:106119 doi: 10.1016/j.phrs.2022.106119
[36] MOREIRA J B N,WOHLWEND M,WISLØFF U. Exercise and cardiac health:Physiological and molecular insights[J]. Nature Metabolism,2020,2(9):829-839 doi: 10.1038/s42255-020-0262-1
[37] DA SILVA V L,MOTA G A F,DE SOUZA S L B,et al. Aerobic exercise training improves calcium handling and cardiac function in rats with heart failure resulting from aortic stenosis[J]. International Journal of Molecular Sciences,2023,24(15):12306 doi: 10.3390/ijms241512306
[38] WERNER C,HANHOUN M,WIDMANN T,et al. Effects of physical exercise on myocardial telomere-regulating proteins,survival pathways,and apoptosis[J]. Journal of the American College of Cardiology,2008,52(6):470-482 doi: 10.1016/j.jacc.2008.04.034
[39] LERCHENMÜLLER C,VUJIC A,MITTAG S,et al. Restoration of cardiomyogenesis in aged mouse hearts by voluntary exercise[J]. Circulation,2022,146(5):412-426 doi: 10.1161/CIRCULATIONAHA.121.057276
[40] MA L J,LI K F,WEI W X,et al. Exercise protects aged mice against coronary endothelial senescence via FUNDC1-dependent mitophagy[J]. Redox Biology,2023,62:102693 doi: 10.1016/j.redox.2023.102693
[41] LI H B,HASTINGS M H,RHEE J,et al. Targeting age-related pathways in heart failure[J]. Circulation Research,2020,126(4):533-551 doi: 10.1161/CIRCRESAHA.119.315889
[42] WU X T,WANG L J,WANG K,et al. ADAR2 increases in exercised heart and protects against myocardial infarction and doxorubicin-induced cardiotoxicity[J]. Molecular Therapy,2022,30(1):400-414 doi: 10.1016/j.ymthe.2021.07.004
[43] BEI Y H,LU D C,BÄR C,et al. miR-486 attenuates cardiac ischemia/reperfusion injury and mediates the beneficial effect of exercise for myocardial protection[J]. Molecular Therapy,2022,30(4):1675-1691 doi: 10.1016/j.ymthe.2022.01.031
[44] ZHOU Q L,DENG J L,YAO J H,et al. Exercise downregulates HIPK2 and HIPK2 inhibition protects against myocardial infarction[J]. eBioMedicine,2021,74:103713 doi: 10.1016/j.ebiom.2021.103713
[45] FANG X X,WANG H,HAN D,et al. Ferroptosis as a target for protection against cardiomyopathy[J]. Proceedings of the National Academy of Sciences of the United States of America,2019,116(7):2672-2680
[46] WANG L,QIAO Y,YU J Z,et al. Endurance exercise preconditioning alleviates ferroptosis induced by doxorubicin-induced cardiotoxicity through mitochondrial superoxide-dependent AMPKα2 activation[J]. Redox Biology,2024,70:103079 doi: 10.1016/j.redox.2024.103079
[47] DONE A J,TRAUSTADÓTTIR T. Nrf2 mediates redox adaptations to exercise[J]. Redox Biology,2016,10:191-199 doi: 10.1016/j.redox.2016.10.003
[48] WANG J,ZHU Q,WANG Y,et al. Irisin protects against sepsis-associated encephalopathy by suppressing ferroptosis via activation of the Nrf2/GPX4 signal axis[J]. Free Radical Biology and Medicine,2022,187:171-184 doi: 10.1016/j.freeradbiomed.2022.05.023
[49] DEL RE D P,AMGALAN D,LINKERMANN A,et al. Fundamental mechanisms of regulated cell death and implications for heart disease[J]. Physiological Reviews,2019,99(4):1765-1817 doi: 10.1152/physrev.00022.2018
[50] XU Z J,MA Z Y,ZHAO X Q,et al. Aerobic exercise mitigates high-fat diet-induced cardiac dysfunction,pyroptosis,and inflammation by inhibiting STING-NLRP3 signaling pathway[J]. Molecular and Cellular Biochemistry,2024,479(12):3459-3470 doi: 10.1007/s11010-024-04950-0
[51] KAR S,SHAHSHAHAN H R,HACKFORT B T,et al. Exercise training promotes cardiac hydrogen sulfide biosynthesis and mitigates pyroptosis to prevent high-fat diet-induced diabetic cardiomyopathy[J]. Antioxidants,2019,8(12):638 doi: 10.3390/antiox8120638
[52] SCIARRETTA S,MAEJIMA Y,ZABLOCKI D,et al. The role of autophagy in the heart[J]. Annual Review of Physiology,2018,80:1-26 doi: 10.1146/annurev-physiol-021317-121427
[53] WANG L J,WANG J Q,CRETOIU D,et al. Exercise-mediated regulation of autophagy in the cardiovascular system[J]. Journal of Sport and Health Science,2020,9(3):203-210 doi: 10.1016/j.jshs.2019.10.001
[54] WANG L J,FENG J Y,FENG X,et al. Exercise-induced circular RNA circUtrn is required for cardiac physiological hypertrophy and prevents myocardial ischaemia–reperfusion injury[J]. Cardiovascular Research,2023,119(16):2638-2652 doi: 10.1093/cvr/cvad161
[55] WANG L J,WANG J Q,YU P J,et al. METTL14 is required for exercise-induced cardiac hypertrophy and protects against myocardial ischemia-reperfusion injury[J]. Nature Communications,2022,13:6762 doi: 10.1038/s41467-022-34434-y
[56] GAO R R,WANG L J,BEI Y H,et al. Long noncoding RNA cardiac physiological hypertrophy–associated regulator induces cardiac physiological hypertrophy and promotes functional recovery after myocardial ischemia-reperfusion injury[J]. Circulation,2021,144(4):303-317 doi: 10.1161/CIRCULATIONAHA.120.050446
[57] MA Y X,KUANG Y X,BO W Y,et al. Exercise training alleviates cardiac fibrosis through increasing fibroblast growth factor 21 and regulating TGF-β1-Smad2/3-MMP2/9 signaling in mice with myocardial infarction[J]. International Journal of Molecular Sciences,2021,22(22):12341 doi: 10.3390/ijms222212341
[58] LI H Z,QIN S G,TANG J,et al. Resistance exercise upregulates Irisin expression and suppresses myocardial fibrosis following myocardial infarction via activating AMPK-Sirt1 and inactivating TGFβ1-Smad2/3[J]. Acta Physiologica,2024,240(7):e14163 doi: 10.1111/apha.14163
[59] XI Y,HAO M L,LIANG Q Q,et al. Dynamic resistance exercise increases skeletal muscle-derived FSTL1 inducing cardiac angiogenesis via DIP2A–Smad2/3 in rats following myocardial infarction[J]. Journal of Sport and Health Science,2021,10(5):594-603 doi: 10.1016/j.jshs.2020.11.010
[60] SONG W,LIANG Q Q,CAI M X,et al. HIF-1α-induced up-regulation of microRNA-126 contributes to the effectiveness of exercise training on myocardial angiogenesis in myocardial infarction rats[J]. Journal of Cellular and Molecular Medicine,2020,24(22):12970-12979 doi: 10.1111/jcmm.15892
[61] CHI C,FU H,LI Y H,et al. Exerkine fibronectin type-Ⅲ domain-containing protein 5/irisin-enriched extracellular vesicles delay vascular ageing by increasing SIRT6 stability[J]. European Heart Journal,2022,43(43):4579-4595 doi: 10.1093/eurheartj/ehac431
[62] RABINOVICH-NIKITIN I,KIRSHENBAUM L A. Exercise-induced myonectin protects against ischemia-reperfusion injury[J]. Circulation Research,2018,123(12):1264-1266 doi: 10.1161/CIRCRESAHA.118.314129
[63] ZHANG N N,WANG X P,FENG M Y,et al. Early-life exercise induces immunometabolic epigenetic modification enhancing anti-inflammatory immunity in middle-aged male mice[J]. Nature Communications,2024,15:3103 doi: 10.1038/s41467-024-47458-3
[64] MURASHIGE D,JANG C,NEINAST M,et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart[J]. Science,2020,370(6514):364-368 doi: 10.1126/science.abc8861
[65] NEUBAUER S. The failing heart:An engine out of fuel[J]. New England Journal of Medicine,2007,356(11):1140-1151 doi: 10.1056/NEJMra063052
[66] JIN L G,GENG L L,YING L,et al. FGF21–sirtuin 3 axis confers the protective effects of exercise against diabetic cardiomyopathy by governing mitochondrial integrity[J]. Circulation,2022,146(20):1537-1557 doi: 10.1161/CIRCULATIONAHA.122.059631
[67] CAMPOS J C,QUELICONI B B,BOZI L H M,et al. Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure[J]. Autophagy,2017,13(8):1304-1317 doi: 10.1080/15548627.2017.1325062
[68] GIBB A A,HILL B G. Metabolic coordination of physiological and pathological cardiac remodeling[J]. Circulation Research,2018,123(1):107-128 doi: 10.1161/CIRCRESAHA.118.312017
[69] RISIKESAN J,HEEBØLL S,KUMARATHAS I,et al. Exercise increases myocardial free fatty acid oxidation in subjects with metabolic dysfunction-associated fatty liver disease[J]. Atherosclerosis,2023,372:10-18 doi: 10.1016/j.atherosclerosis.2023.03.015
[70] JIANG H,JIA D L,ZHANG B J,et al. Exercise improves cardiac function and glucose metabolism in mice with experimental myocardial infarction through inhibiting HDAC4 and upregulating GLUT1 expression[J]. Basic Research in Cardiology,2020,115(3):28 doi: 10.1007/s00395-020-0787-1
[71] FULGHUM K L,COLLINS H E,LORKIEWICZ P K,et al. Exercise-induced changes in myocardial glucose utilization during periods of active cardiac growth[J]. Journal of Molecular and Cellular Cardiology,2024,191:50-62 doi: 10.1016/j.yjmcc.2024.04.014
[72] SATTLER S,CAMPOS RAMOS G,LUDEWIG B,et al. Cardioimmunology:The new frontier![J]. European Heart Journal,2023,44(26):2355-2357 doi: 10.1093/eurheartj/ehad230
[73] SWIRSKI F K,NAHRENDORF M. Cardioimmunology:The immune system in cardiac homeostasis and disease[J]. Nature Reviews Immunology,2018,18:733-744 doi: 10.1038/s41577-018-0065-8
[74] RURIK J G,AGHAJANIAN H,EPSTEIN J A. Immune cells and immunotherapy for cardiac injury and repair[J]. Circulation Research,2021,128(11):1766-1779 doi: 10.1161/CIRCRESAHA.121.318005
[75] MANN D L. The emerging field of cardioimmunology:Past,present and foreseeable future[J]. Circulation Research,2024,134(12):1663-1680 doi: 10.1161/CIRCRESAHA.123.323656
[76] SEVERINSEN M C K,PEDERSEN B K. Muscle–organ crosstalk:The emerging roles of myokines[J]. Endocrine Reviews,2020,41(4):594-609 doi: 10.1210/endrev/bnaa016
[77] TU Y M,LIU J Z,KONG D Q,et al. Irisin drives macrophage anti-inflammatory differentiation via JAK2-STAT6-dependent activation of PPARγ and Nrf2 signaling[J]. Free Radical Biology and Medicine,2023,201:98-110 doi: 10.1016/j.freeradbiomed.2023.03.014
[78] JITMANA R,RAKSAPHARM S,KIJTAWORNRAT A,et al. Role of cardiac mast cells in exercise training-mediated cardiac remodeling in angiotensin Ⅱ-infused ovariectomized rats[J]. Life Sciences,2019,219:209-218 doi: 10.1016/j.lfs.2019.01.018
[79] SUN M Y,MAO S,WU C,et al. Piezo1-mediated neurogenic inflammatory cascade exacerbates ventricular remodeling after myocardial infarction[J]. Circulation,2024,149(19):1516-1533 doi: 10.1161/CIRCULATIONAHA.123.065390
[80] PENG H,HU B,XIE L Q,et al. A mechanosensitive lipolytic factor in the bone marrow promotes osteogenesis and lymphopoiesis[J]. Cell Metabolism,2022,34(8):1168-1182. e1-e6
[81] HAN M S,WHITE A,PERRY R J,et al. Regulation of adipose tissue inflammation by interleukin 6[J]. Proceedings of the National Academy of Sciences of the United States of America,2020,117(6):2751-2760
[82] PAN J N,ZHANG H,LIN H,et al. Irisin ameliorates doxorubicin-induced cardiac perivascular fibrosis through inhibiting endothelial-to-mesenchymal transition by regulating ROS accumulation and autophagy disorder in endothelial cells[J]. Redox Biology,2021,46:102120 doi: 10.1016/j.redox.2021.102120
[83] OTAKA N,SHIBATA R,OHASHI K,et al. Myonectin is an exercise-induced myokine that protects the heart from ischemia-reperfusion injury[J]. Circulation Research,2018,123(12):1326-1338 doi: 10.1161/CIRCRESAHA.118.313777
[84] HUANG Y H,WANG S B,ZHOU J,et al. IRF1-mediated downregulation of PGC1α contributes to cardiorenal syndrome type 4[J]. Nature Communications,2020,11:4664 doi: 10.1038/s41467-020-18519-0
[85] FLORENS N,KASAM R K,RUDMAN-MELNICK V,et al. Interleukin-33 mediates cardiomyopathy after acute kidney injury by signaling to cardiomyocytes[J]. Circulation,2023,147(9):746-758 doi: 10.1161/CIRCULATIONAHA.122.063014
[86] YANG P R,READ C,KUC R E,et al. Elabela/toddler is an endogenous agonist of the apelin APJ receptor in the adult cardiovascular system,and exogenous administration of the peptide compensates for the downregulation of its expression in pulmonary arterial hypertension[J]. Circulation,2017,135(12):1160-1173 doi: 10.1161/CIRCULATIONAHA.116.023218
[87] SATO T,SATO C,KADOWAKI A,et al. ELABELA-APJ axis protects from pressure overload heart failure and angiotensin Ⅱ-induced cardiac damage[J]. Cardiovascular Research,2017,113(7):760-769 doi: 10.1093/cvr/cvx061
[88] ZHANG Z Z,TANG J Q,SONG J W,et al. Elabela alleviates ferroptosis,myocardial remodeling,fibrosis and heart dysfunction in hypertensive mice by modulating the IL-6/STAT3/GPX4 signaling[J]. Free Radical Biology and Medicine,2022,181:130-142 doi: 10.1016/j.freeradbiomed.2022.01.020
[89] XI Y,LI Y X,REN W J,et al. ELABELA-APJ-Akt/YAP signaling axis:A novel mechanism of aerobic exercise in cardioprotection of myocardial infarction rats[J]. Medicine & Science in Sports & Exercise,2023,55(7):1172-1183
[90] DAI Y X,SUN Z H,ZHENG Y,et al. Recent advances in the gut microbiome and microbial metabolites alterations of coronary artery disease[J]. Science Bulletin,2023,68(6):549-552 doi: 10.1016/j.scib.2023.03.009
[91] O'BRIEN M T,O'SULLIVAN O,CLAESSON M J,et al. The athlete gut microbiome and its relevance to health and performance:A review[J]. Sports Medicine,2022,52(1):119-128
[92] TANG T W H,CHEN H C,CHEN C Y,et al. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair[J]. Circulation,2019,139(5):647-659 doi: 10.1161/CIRCULATIONAHA.118.035235
[93] FIUZA-LUCES C,SANTOS-LOZANO A,JOYNER M,et al. Exercise benefits in cardiovascular disease:Beyond attenuation of traditional risk factors[J]. Nature Reviews Cardiology,2018,15:731-743 doi: 10.1038/s41569-018-0065-1
[94] WITKOWSKI M,WEEKS T L,HAZEN S L. Gut microbiota and cardiovascular disease[J]. Circulation Research,2020,127(4):553-570 doi: 10.1161/CIRCRESAHA.120.316242
[95] ZHOU Q L,DENG J L,PAN X,et al. Gut microbiome mediates the protective effects of exercise after myocardial infarction[J]. Microbiome,2022,10(1):82 doi: 10.1186/s40168-022-01271-6
[96] MANTOVANI A,BYRNE C D,BENFARI G,et al. Risk of heart failure in patients with nonalcoholic fatty liver disease JACC review topic of the week[J]. Journal of the American College of Cardiology,2022,79(2):180-191 doi: 10.1016/j.jacc.2021.11.007
[97] CHEN M,ZHU J Y,MU W J,et al. Cdo1-Camkk2-AMPK axis confers the protective effects of exercise against NAFLD in mice[J]. Nature Communications,2023,14:8391 doi: 10.1038/s41467-023-44242-7
[98] WANG T,YU M Y,LI H Z,et al. FNDC5/irisin inhibits the inflammatory response and mediates the aerobic exercise-induced improvement of liver injury after myocardial infarction[J]. International Journal of Molecular Sciences,2023,24(4):4159 doi: 10.3390/ijms24044159
[99] LOU J,WU J,FENG M Y,et al. Exercise promotes angiogenesis by enhancing endothelial cell fatty acid utilization via liver-derived extracellular vesicle miR-122-5p[J]. Journal of Sport and Health Science,2022,11(4):495-508 doi: 10.1016/j.jshs.2021.09.009
[100] BO W Y,MA Y X,FENG L L,et al. FGF21 promotes myocardial angiogenesis and mediates the cardioprotective effects of exercise in myocardial infarction mice[J]. Journal of Applied Physiology,2023,135(3):696-705 doi: 10.1152/japplphysiol.00307.2023
[101] JIN L G,DIAZ-CANESTRO C,WANG Y,et al. Exerkines and cardiometabolic benefits of exercise:From bench to clinic[J]. EMBO Molecular Medicine,2024,16(3):432-444 doi: 10.1038/s44321-024-00027-z
[102] TANG T T,LI Y Y,LI J J,et al. Liver-heart crosstalk controls IL-22 activity in cardiac protection after myocardial infarction[J]. Theranostics,2018,8(16):4552-4562
[103] PACKER M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium[J]. Journal of the American College of Cardiology,2018,71(20):2360-2372 doi: 10.1016/j.jacc.2018.03.509
[104] AHMAD I,GUPTA S,FAULKNER P,et al. Single-nucleus transcriptomics of epicardial adipose tissue from female pigs reveals effects of exercise training on resident innate and adaptive immune cells[J]. Cell Communication and Signaling,2024,22(1):243 doi: 10.1186/s12964-024-01587-w
[105] CHO D H,PARK S M. Epicardial adipose tissue and heart failure,friend or foe?[J]. Diabetes & Metabolism Journal,2024,48(3):373-384
[106] ZHAO S G,KUSMINSKI C M,SCHERER P E. Adiponectin,leptin and cardiovascular disorders[J]. Circulation Research,2021,128(1):136-149 doi: 10.1161/CIRCRESAHA.120.314458
[107] KHALAFI M,HOSSEIN SAKHAEI M,KHERADMAND S,et al. The impact of exercise and dietary interventions on circulating leptin and adiponectin in individuals who are overweight and those with obesity:A systematic review and meta-analysis[J]. Advances in Nutrition,2023,14(1):128-146 doi: 10.1016/j.advnut.2022.10.001
[108] HU J G,LIU T,FU F,et al. Omentin1 ameliorates myocardial ischemia-induced heart failure via SIRT3/FOXO3a-dependent mitochondrial dynamical homeostasis and mitophagy[J]. Journal of Translational Medicine,2022,20(1):447 doi: 10.1186/s12967-022-03642-x
[109] GAO K L,SU Z G,MENG J Y,et al. Effect of exercise training on some anti-inflammatory adipokines,high sensitivity C-reactive protein,and clinical outcomes in sedentary adults with metabolic syndrome[J]. Biological Research for Nursing,2024,26(1):125-138 doi: 10.1177/10998004231195541
[110] MACHHADA A,HOSFORD P S,DYSON A,et al. Optogenetic stimulation of vagal efferent activity preserves left ventricular function in experimental heart failure[J]. JACC:Basic to Translational Science,2020,5(8):799-810 doi: 10.1016/j.jacbts.2020.06.002
[111] PARRY-WILLIAMS G,SHARMA S. The effects of endurance exercise on the heart:Panacea or poison?[J]. Nature Reviews Cardiology,2020,17:402-412 doi: 10.1038/s41569-020-0354-3
[112] LI Z X,LI Z,XU W G,et al. The connectome from the cerebral cortex to the viscera using viral transneuronal tracers[J]. American Journal of Translational Research,2021,13(11):12152-12167
[113] BO W Y,CAI M X,MA Y X,et al. Manipulation of glutamatergic neuronal activity in the primary motor cortex regulates cardiac function in normal and myocardial infarction mice[J]. Advanced Science,2024,11(20):2305581 doi: 10.1002/advs.202305581
[114] MA A Y,HONG J,SHANKS J,et al. Upregulating Nrf2 in the RVLM ameliorates sympatho-excitation in mice with chronic heart failure[J]. Free Radical Biology and Medicine,2019,141:84-92 doi: 10.1016/j.freeradbiomed.2019.06.002
[115] WAFI A M,YU L,GAO L,et al. Exercise training upregulates Nrf2 protein in the rostral ventrolateral medulla of mice with heart failure[J]. Journal of Applied Physiology,2019,127(5):1349-1359 doi: 10.1152/japplphysiol.00469.2019
[116] HIGASHIKUNI Y,LIU W H,NUMATA G,et al. NLRP3 inflammasome activation through heart-brain interaction initiates cardiac inflammation and hypertrophy during pressure overload[J]. Circulation,2023,147(4):338-355 doi: 10.1161/CIRCULATIONAHA.122.060860
[117] CHOI J W,JO S W,KIM D E,et al. Aerobic exercise attenuates LPS-induced cognitive dysfunction by reducing oxidative stress,glial activation,and neuroinflammation[J]. Redox Biology,2024,71:103101 doi: 10.1016/j.redox.2024.103101
[118] FENG L L,LI B W,CAI M X,et al. Resistance exercise alleviates the prefrontal lobe injury and dysfunction by activating SESN2/AMPK/PGC-1α signaling pathway and inhibiting oxidative stress and inflammation in mice with myocardial infarction[J]. Experimental Neurology,2023,370:114559 doi: 10.1016/j.expneurol.2023.114559
[119] GIANNONI A,GENTILE F,BUONCRISTIANI F,et al. Chemoreflex and baroreflex sensitivity hold a strong prognostic value in chronic heart failure[J]. JACC:Heart Failure,2022,10(9):662-676
[120] BLUMENTHAL J A,HINDERLITER A L,SMITH P J,et al. Effects of lifestyle modification on patients with resistant hypertension:Results of the TRIUMPH randomized clinical trial[J]. Circulation,2021,144(15):1212-1226 doi: 10.1161/CIRCULATIONAHA.121.055329
[121] DÍAZ-JARA E,DÍAZ H S,RIOS-GALLARDO A,et al. Exercise training reduces brainstem oxidative stress and restores normal breathing function in heart failure[J]. Free Radical Biology and Medicine,2021,172:470-481 doi: 10.1016/j.freeradbiomed.2021.06.032
[122] REDFIELD M M,BORLAUG B A. Heart failure with preserved ejection fraction[J]. JAMA,2023,329(10):827 doi: 10.1001/jama.2023.2020
[123] MENG G N,ZHOU X Y,WANG M L,et al. Gut microbe-derived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways[J]. eBioMedicine,2019,44:656-664 doi: 10.1016/j.ebiom.2019.03.066
[124] BATTILLO D J,MALIN S K. Impact of caloric restriction and exercise on trimethylamine N-oxide metabolism in women with obesity[J]. Nutrients,2023,15(6):1455 doi: 10.3390/nu15061455
[125] XIA W J,XU M L,YU X J,et al. Antihypertensive effects of exercise involve reshaping of gut microbiota and improvement of gut-brain axis in spontaneously hypertensive rat[J]. Gut Microbes,2021,13(1):1-24
[126] MCCRACKEN C,RAISI-ESTABRAGH Z,VELDSMAN M,et al. Multi-organ imaging demonstrates the heart-brain-liver axis in UK Biobank participants[J]. Nature Communications,2022,13:7839 doi: 10.1038/s41467-022-35321-2
[127] JIN Q,YANG R X,FAN J G. Does nonalcoholic fatty liver disease predispose patients to carotid arteriosclerosis and ischemic stroke?[J]. Clinical and Molecular Hepatology,2022,28(3):473-477 doi: 10.3350/cmh.2022.0155
[128] HERMANNS N,WROBLEWSKI V,BASCUÑANA P,et al. Molecular imaging of the brain–heart axis provides insights into cardiac dysfunction after cerebral ischemia[J]. Basic Research in Cardiology,2022,117(1):52 doi: 10.1007/s00395-022-00961-4
[129] WU Y,DONG J H,DAI Y F,et al. Hepatic soluble epoxide hydrolase activity regulates cerebral Aβ metabolism and the pathogenesis of Alzheimer's disease in mice[J]. Neuron,2023,111(18):2847-2862. e10
[130] STAKOS D A,STAMATELOPOULOS K,BAMPATSIAS D,et al. The Alzheimer's disease amyloid-beta hypothesis in cardiovascular aging and disease JACC focus seminar[J]. Journal of the American College of Cardiology,2020,75(8):952-967 doi: 10.1016/j.jacc.2019.12.033
[131] WRANN C D,WHITE J P,SALOGIANNNIS J,et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway[J]. Cell Metabolism,2013,18(5):649-659 doi: 10.1016/j.cmet.2013.09.008
[132] BAI P Y,CHEN S Q,JIA D L,et al. Environmental eustress improves postinfarction cardiac repair via enhancing cardiac macrophage survival[J]. Science Advances,2022,8(17):eabm3436 doi: 10.1126/sciadv.abm3436
[133] CHOI M,SCHNEEBERGER M,FAN W,et al. FGF21 counteracts alcohol intoxication by activating the noradrenergic nervous system[J]. Cell Metabolism,2023,35(3):429-437. e5
[134] HAYKIN H,AVISHAI E,KROT M,et al. Reward system activation improves recovery from acute myocardial infarction[J]. Nature Cardiovascular Research,2024,3:841-856 doi: 10.1038/s44161-024-00491-3
[135] LI J L,SUN S H,ZHU D S,et al. Inhalable stem cell exosomes promote heart repair after myocardial infarction[J]. Circulation,2024,150(9):710-723 doi: 10.1161/CIRCULATIONAHA.123.065005
[136] TAN Y Z,FENG P,FENG L L,et al. Low-dose exercise protects the heart against established myocardial infarction via IGF-1-upregulated CTRP9 in male mice[J]. MedComm,2023,4(6):e411 doi: 10.1002/mco2.411
[137] YIN A W,YUAN R S,XIAO Q Q,et al. Exercise-derived peptide protects against pathological cardiac remodeling[J]. eBioMedicine,2022,82:104164 doi: 10.1016/j.ebiom.2022.104164
[138] FENG L F,LI G R,AN J L,et al. Exercise training protects against heart failure via expansion of myeloid-derived suppressor cells through regulating IL-10/STAT3/S100A9 pathway[J]. Circulation:Heart Failure,2022,15(3):e008550 doi: 10.1161/CIRCHEARTFAILURE.121.008550
[139] LI H Z,QIN S G,LIANG Q Q,et al. Exercise training enhances myocardial mitophagy and improves cardiac function via irisin/FNDC5-PINK1/parkin pathway in MI mice[J]. Biomedicines,2021,9(6):701 doi: 10.3390/biomedicines9060701
[140] LIN H R,ZHU Y Q,ZHENG C K,et al. Antihypertrophic memory after regression of exercise-induced physiological myocardial hypertrophy is mediated by the long noncoding RNA Mhrt779[J]. Circulation,2021,143(23):2277-2292 doi: 10.1161/CIRCULATIONAHA.120.047000
[141] ZHU Y Q,ZHENG C K,ZHANG R,et al. Circ-Ddx60 contributes to the antihypertrophic memory of exercise hypertrophic preconditioning[J]. Journal of Advanced Research,2023,46:113-121 doi: 10.1016/j.jare.2022.06.005
[142] SHI J,BEI Y H,KONG X Q,et al. miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury[J]. Theranostics,7(3):664-676
[143] AMAR D,GAY N R,JEAN-BELTRAN P M,et al. Temporal dynamics of the multi-omic response to endurance exercise training[J]. Nature,2024,629:174-183 doi: 10.1038/s41586-023-06877-w
[144] VETR N G,GAY N R,ADKINS J N,et al. The impact of exercise on gene regulation in association with complex trait genetics[J]. Nature Communications,2024,15:3346 doi: 10.1038/s41467-024-45966-w
[145] MCDONAGH S T,DALAL H,MOORE S,et al. Home-based versus centre-based cardiac rehabilitation[J]. Cochrane Database of Systematic Reviews,2023,2023(10):Cd007130
[146] LU J,LIU J J,ZHANG L M,et al. Morphological and functional characterization of diabetic cardiomyopathy in db/db mice following exercise,metformin alone,or combination treatments[J]. Biochemical and Biophysical Research Communications,2021,584:80-86 doi: 10.1016/j.bbrc.2021.11.018
[147] ISMAIL H,MCFARLANE J R,NOJOUMIAN A H,et al. Clinical outcomes and cardiovascular responses to different exercise training intensities in patients with heart failure: A systematic review and meta-analysis[J]. JACC:Heart Failure,2013,1(6):514-522
[148] LAOUTARIS I D,PIOTROWICZ E,KALLISTRATOS M S,et al. Combined aerobic/resistance/inspiratory muscle training as the 'optimum' exercise programme for patients with chronic heart failure:ARISTOS-HF randomized clinical trial[J]. European Journal of Preventive Cardiology,2021,28(15):1626-1635 doi: 10.1093/eurjpc/zwaa091
[149] GOMES-NETO M,DURÃES A R,CONCEIÇÃO L S R,et al. Some types of exercise interventions are more effective than others in people with coronary heart disease:Systematic review and network meta-analysis[J]. Journal of Physiotherapy,2024,70(2):106-114 doi: 10.1016/j.jphys.2024.02.018
[150] CHRISTENSEN R H,WEDELL-NEERGAARD A S,LEHRSKOV L L,et al. Effect of aerobic and resistance exercise on cardiac adipose tissues[J]. JAMA Cardiology,2019,4(8):778 doi: 10.1001/jamacardio.2019.2074




 下载:
下载: